| A | B |
|---|
| What are the building blocks of all matter? | atoms p. 33 |
| The nucleus of an atom is made up of ____ and ____ | protons and neutrons p. 33 |
| Electrons have a ___ charge | negative p. 33 |
| Protons have a ___ charge | positive p. 33 |
| Neutrons have a ___ charge | neutral (no) charge p. 33 |
| Which type of sub-particle orbits the nucleus? | electrons p. 33 |
| Positive charges are attracted to ____ charges | negative p. 33 |
| Positive charges are repelled by ____ charges. | positive p. 33 |
| Negative charges are repelled by ____ charges | negative p. 33 |
| An electron would be attracted to which type of sub-atomic particle? | proton p. 33 |
| The center region of an atom is called a(n) ____. | atomic nucleus p. 33 |
| Each element is different because they have a different number of ____. | protons p. 33, 34 |
| All of the elements are listed in the ____. | periodic table p. 36 and appendix B |
| The ____ is equal to the number of protons in an atom. | atomic number p. 33 |
| The number of protons + the number of neutrons is equal to the ____ | mass number p. 33 |
| The mass number is equal to the | number of protons and neutrons p. 33 |
| The atomic number is equal to the number of ____ in an atom | protons p. 33 |
| The number below the chemical symbol on the periodic table that usually has a few decimal places is known as the ____. | average atomic mass p. 34 |
| If an element has a mass # of 23 and an atomic # of 11, how many protons will it have? Neutrons? | 11 protons and 12 neutrons p. 33 |
| Two or more different elements bond together to form ____. | compounds p. 31 |
| A compound held together by covalent bonds is called a(n) ___. | molecule p. 38 |
| Atoms or molecules that become charged because they gain or lose electrons are called ___. | ions p. 40 |
| A negatively charged ion would be ____ by another negatively charged ion. | repelled p. 40 |
| A positively charged ion would be ____ by another positively charged ion. | repelled p. 40 |
| A negatively charged ion would be ___ by a positively charged ion. | attracted p. 40 |
| Matter is _____. | Anything that takes up space and has mass. p. 31 |
| _____ is anything that takes up space and has mass. | Matter p. 31 |
| A(n) ______ is a substance that cannot be broken down to other substances by chemical reactions. | element p. 31 |
| Two types of compounds are ____ and ____. | molecules and ionic compounds. pp.. 38&40 |
| A compound in which atoms are joined together by ionic bonds is called a(n) ___. | ionic compound p. 40 |
| Give an example of a molecule that is not a compound. | Any diatomic molecule, like O2, or H2, or N2, is a molecule because the two atoms are joined by covalent bonds, but is not a compound because they aren't made of two or move DIFFERENT elements. Diatomic molecules are called pure elements. p. 38 |
| Which 4 elements make up 96% of living matter? | Carbon, Hydrogen, Oxygen, and Nitrogen p. 32 |
| Elements that are only required by organisms in minute quantities are called _____. | trace elements p. 32 |
| A deficiency of the trace element ____ in the diet can lead to an enlargement of the thyroid. This enlargement is called a(n) _____. | iodine, goiter (the enlarged part of the neck in the picture below is the goiter) p. 32,  |
| A(n) ___ is the smallest unit of matter that still retains the properties of an element. | atom p. G3 |
| The tiny bits of matter that make up an atom are called ___. | subatomic particles p. 33 |
| Protons and neutrons each have a mass of 1 _____ (a.k.a. _____) | dalton, atomic mass unit (amu) p. 33 |
| Isotopes of a certain element have the same number of ____ but differ in the number of _____. | protons, neutrons p. 34 |
| Elements that differ from each other because of the number neutrons, but not protons, are called ____. | isotopes p. 34 |
| Scientists use _____ to label certain chemical substances, creating tracers that can be used to follow a metabolic process or locate the substance within an organism. | radioactive isotopes p. 34 |
| _____ is defined as the capacity to cause change. | Energy p. 35 |
| ____ is the energy that matter possesses due to its location or structure. | Potential energy p. 35 |
| Matter has a natural tendency to move to the ____ possible state of potential energy. | lowest p. 35 |
| Electrons gain potential energy when they move ____ from the nucleus. | away p. 35 |
| When an electron falls back toward the nucleus, energy is ___. | released (or lost) p. 35 |
| The different states of potential energy that electrons have in an atom are called _____ and are represented by ____. | energy levels, electron shells p. 35 |
| When electrons lose energy (by falling back toward the nucleus), the lost energy is usually released to the environment in the form of ___. | heat p. 35 |
| The first electron shell of an atom can hold no more than ___ electrons. | two p.36 |
| The second electron shell of an atom can hold no more than ___ electrons. | eight p.36 |
| The chemical behavior of an element depends mostly on ____. | the number of electrons in its outermost shell |
| Electrons in the outermost shell of an atom are called ____. | valence electrons p. 36 |
| The outermost shell of an atom is called the ___. | valence shell p. 36 |
| An atom with a full valence shell of electrons is chemically _____ (a.k.a. _____) | unreactive, inert p. 36 |
| The three-dimensional space where an electron can be found 90% of the time is called a(n) ___. | orbital p. 37 |
| A maximum of ___ electrons can be found in any one orbital. | 2 (Don't get orbitals mixed up with shells or energy levels. For instance, the second energy level has 4 orbitals. One s-orbital and three p-orbitals) p. 37 |
| Spherical orbitals are called ____ and there is/are ____ of these at each energy level. | s-orbital, one p. 37,  |
| Dumbbell shaped orbitals are called ____ and there is/are ____ of these at each energy level. | p-orbital, 3 at each energy level except the first which has none p. 37,  |
| The two strongest types of chemical bonds are _____ and _______ bonds. | covalent, ionic p. 38 |
| In _____ bonds, pairs of electrons are shared between two or more atoms. | covalent p. 38 |
| Single covalent bonds share ___ pair(s) of electrons. | one pair (two electrons total) p. 38 |
| Double covalent bonds share ___ pair(s) of electrons. | two pairs (4 electrons total) p. 38 |
| Triple covalent bonds share ___ pair(s) of electrons. | three pairs (6 electrons total) p. 38 inferred |
| Write the molecular formula for a molecule of oxygen. | p. 38,  |
| Write the structural formula for a molecule of oxygen. | p. 38,  |
| Draw the electron distribution diagram for a molecule of oxygen. | p. 38,  |
| The number of covalent bonds that an atom can form is called its _______ or ________ and is usually equal to the number of ______ in the atoms outermost shell. | bonding capacity or valence, usually equal to the number of unpaired electrons in the atoms outermost (valence) shell. p. 39 |
| The attraction of a particular kind of atom for the electrons of a covalent bond is called its ___. | electronegativity p. 39 |
| The more ____ an atom is, the more strongly it pulls shared electrons toward itself. | electronegative p. 39 |
| The _____ electronegative an atom is, the more strongly it pulls shared electrons toward itself. | more p. 39 |
| Bonds in which the electrons between two atoms are shared about equally (because both atoms have similar electronegativities) are called _____. | nonpolar covalent bonds p. 39 |
| Bonds in which the electrons between two atoms are not SHARED equally (because one atom has a significantly higher electronegativity) are called _____. | polar covalent bond p. 39 |
| Bonds in which electrons aren't shared at all (because one atom has a MUCH higher electronegativity than the other) are called ______. | ionic bonds p. 40 |
| A charged atom, (or molecule) is called a(n) ___. | ion p. 40 |
| Positively charged ions are called ____. | cations p. 40 |
| Negatively charged ions are called ____. | anions p. 40 |
| Compounds formed by ionic bonds are called ____ . | ionic compounds p. 40 |
| The most important characteristic about a biological molecule is its ____. | shape p. 42 |
| The s-orbital and 3 p-orbitals hybidize to form the shape of a ______. This is important because it helps explain why bonds coming off many atoms go off at the angles found in this shape. | tetrahedron p. 41, 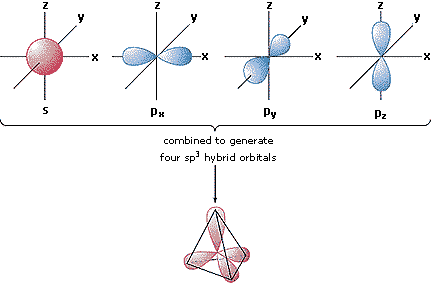 |
In the chemical reaction picture below, the molecules on the left side of arrow are called the ____ while the molecules on the right side of the arrow are called the ____., 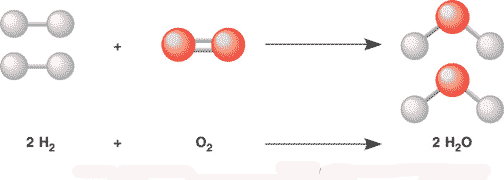 | reactants, products (notice how there are the same number of each type of atom on both sides of this balanced chemical reaction),  |
The picture below shows the formation of a(n) _____ bond.,  | ionic p. 40,  |
| A chemical reaction in which arrows are pointing both ways is said to be ____. | reversible p. 43 |
| In a reversible reaction, when the forward and reverse reactions are happening at the same rate, _____ is achieved. | chemical equilibrium p. 43 |
| True or false: When a reversible chemical reaction reaches equilibrium, the reactants and products are equal in concentration. | False. All it means is that the concentration of reactants and products has stabilized at some fixed ratio. For example, pure distilled water occasionally breaks down into a hydrogen ion and a hydroxide ion. The hydroxide ions almost instantaneously react with the hydrogen ions to reform water. At any one moment, at equilibrium, there are 554 million times more water molecules than there are hydrogen or hydroxide ions in a solution of distilled water (at 25 Celsius) p. 43 |
| Name 2 types of weak bonds in order from strongest to weakest. | hydrogen, London Dispersion forces (weakest): Your book calls London Dispersion forces Van der Waals forces, but Van der Waals forces are actually any type of intermolecular force, including hydrogen bonds. Your book also says that ionic bonds are weak bonds, but makes the distinction that they are weak when the substance is in water, like table salt dissolved in water. pp. 40&41 |
| Hydrogen bonds usually form between hydrogen atoms involved in a polar covalent bond and _____ or _____ atoms in another molecule. | oxygen, nitrogen p. 40 |
| A weak attraction between hydrogen in one molecule and either an oxygen or nitrogen atom in another molecule is called a(n) _____. | hydrogen bond p. 40 |
| A very weak bond that can form between a non-polar region of a molecule and another molecule (as long as the molecules are very close together) is called a(n) ____. | London Dispersion Force (Your book calls London Dispersion forces Van der Waals forces, but Van der Waals forces are actually any type of intermolecular force, including hydrogen bonds. London Dispersion forces can also form between single atoms like the noble gases if you drop the temperature low enough) pp. 40&41 |
| London Dispersion Forces are very weak attractions between two molecules and involve _____ regions. | non-polar (Your book incorrectly calls London Dispersion forces van der Waals forces. London Dispersion forces are the weakest type of Van der Waals force, involving instantaneous induced dipoles between two non-polar substances) pp. 40&41 |
| The type of bond that allows certain geckos to walk up walls is called a(n) _____ | London Dispersion Force (Your book incorrectly calls London Dispersion forces van der Waals forces) pp. 40&41 |
______ bonds allow the endorphin receptors to temporarily bond to morphine or your bodies own natural endorphins because the shapes of the molecules are ____.,  | weak, complementary p. 42,  |
| The root word "an-" means ___. | not (anion: a negatively charged ion) |
| The root word "co-" means ___. | co- = together; -valent = strength (covalent bond: an attraction between atoms that share one or more pairs of outer-shell electrons) |
| The root word "iso-" means ___. | iso- = equal (isotope: an element having the same number of protons and electrons but a different number of neutrons) |
| The root word "neutr-" means ___. | neutr- = neither (neutron: a subatomic particle with a neutral electrical charge) |