|
|
|
|
Stoichiometry
Tutorial
Using "THE MAP" to Solve Problems
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chemists are often responsible for designing
a chemical reaction and analyzing the products obtained from it. One significant
part of designing a chemical reaction is determining the amount of each
reactant that is needed and the amount of each product that will be produced.
In order to do so, chemists use stoichiometry,
the quantitative relationships between reactants and products.
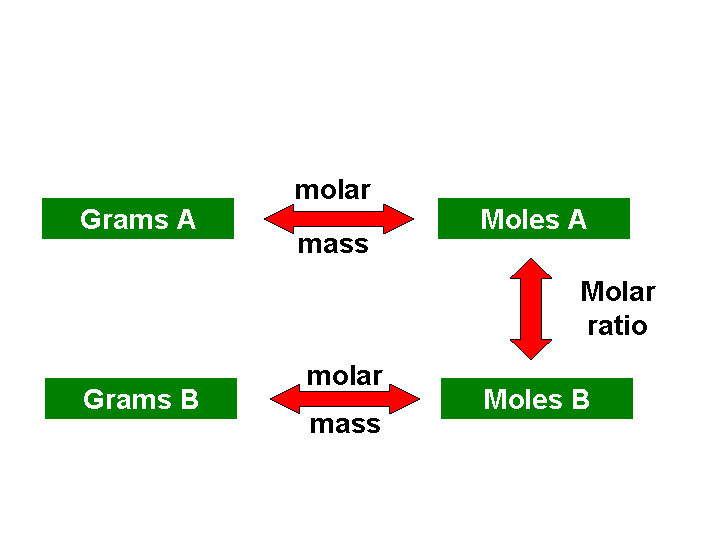
"THE MAP" shown below is your best
friend when it comes to solving stoichiometry problems. Whether you need
to convert from mass of one substance to mass of another substance, from
moles of one substance to moles of a different substance, or simply from
grams of one substance to moles of that same substance, "THE MAP"
tells you exactly how many (and which) steps you will have to use and
which conversion factors you will need. Make your life simpler......memorize
"THE MAP!"
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Solving a stoichiometry problem using "THE
MAP" is kind of like planning a trip. If you were planning a trip
from Oklahoma City to Chicago, you would first find OKC on the map and
then follow the major highways to Tulsa, Springfield, MO, St. Louis, and
then Chicago. You have to do the same thing when using "THE MAP"
to solve a stoichiometry problem. First, find your starting point on the
map. Next, find your ultimate destination on the map. Finally, "simply"
follow the steps outlined on the map to get where you want to be!
Let's work through a couple of examples to
see how "THE MAP" is used to solve stoichiometry problems.
|
|
|
|
|
Converting from Moles
A to Grams of B
|
|
|
|
|
Example:
How many grams of water will be produced from the combustion of 0.152 mol
of propane (C3H8)?
C3H8
(g) + 5 O2 (g) --> 3 CO2 (g) + 4 H2O
(l)
|
|
|
|
|
The first step in solving this problem is
to use "THE MAP" to plan your "trip" (i.e. decide
where you're starting and where you will be when you've solved the problem).
THE MAP for this problem looks like this:
|
|
|
|
|
|

|
|
|
|
|
|
|
|
"THE MAP" tells you that you need
exactly two steps:
-
First, use the molar ratio to convert
mol C3H8 to mol H2O
-
Second, use the molar mass of water to
convert mol H2O to grams H2O.
-
Write down the units
you're looking for (including the formula for the compound) and put
an equal sign.
-
Write the information
you were given in the problem (in
this case, the number of moles of propane). Don't forget to include
the formula for the compound you're starting with:
-
Put a multiplication
sign after the number of moles and draw a line.
| g H2O |
= |
0.152
mol C3H8 |
x |
__________ |
| |
|
|
|
|
-
Above and below the line,
write the molar ratio. Since you
want to get rid of the units you started with (in this case "mol
C3H8), put the units "mol" and the
formula of the compound you started with on the bottom. Put the units
"mol" and the formula of the compound you're looking for
on the top.
| g H2O |
= |
0.152
mol C3H8 |
x |
mol
H2O |
| |
|
|
|
mol C3H8 |
-
Use the coefficients
from the balanced chemical equation to complete the molar ratio.
Place the coefficient in front of the formula that you started with
on the bottom and the coefficient in front of the formula that you
are looking for on the top.
| g H2O |
= |
0.152
mol C3H8 |
x |
4 mol
H2O |
| |
|
|
|
1 mol C3H8 |
-
Cancel out your units
and compare them to the ones you want to finish with.
Since they are not the same, put a multiplication sign after the molar
ratio and draw a line.
| g H2O |
= |
0.152 mol C3H8 |
x |
4 mol
H2O |
x |
_______ |
| |
|
|
|
1 mol C3H8 |
|
|
-
Write the molar mass
above and below the line so that
the mol of water cancel out leaving you with grams of water.
| g H2O |
= |
0.152 mol C3H8 |
x |
4 mol
H2O |
x |
18.0 g H2O |
| |
|
|
|
1 mol C3H8 |
|
1 mol H2O |
-
Cancel out your units
and do the math. Report the answer
with the correct units and the correct number of significant figures.
| g H2O |
= |
0.152 mol C3H8 |
x |
4 mol
H2O |
x |
18.0 g H2O |
| |
|
|
|
1 mol C3H8 |
|
1 mol H2O |
| g H2O |
= |
10.9 g H2O |
|
|
|
|
|
|
|
|
|
Converting From Grams
of A to Grams of B
|
|
|
|
|
Example: How
many grams of CO2
will be produced by the combustion of 15.0 g of propane?
C3H8
(g) + 5 O2 (g) --> 3 CO2 (g) + 4 H2O
(l)
|
|
|
|
|
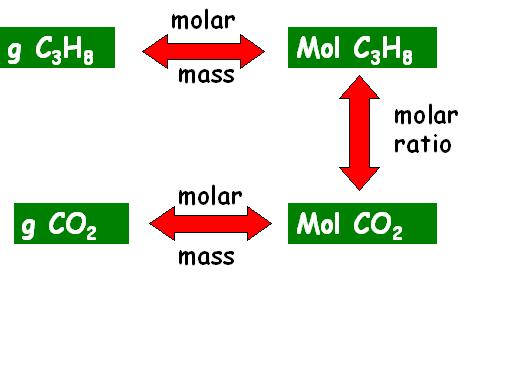
The first step in solving this problem is
to use "THE MAP" to plan your "trip" (i.e. decide
where you're starting and where you will be when you've solved the problem).
THE MAP for this problem looks like this:
|
|
|
|
|
|
|
 |
|
|
|
|
|
|
|
To solve this problem, you will need exactly
three steps:
-
First, use the molar mass to convert
grams C3H8 to moles C3H8
-
Second, use a molar ratio to convert
moles C3H8 to moles CO2
-
Third, use molar mass to convert moles
of CO2
to grams of CO2
-
Write down the units
you're looking for (including the formula for the compound) and put
an equal sign.
-
Write the information
you were given in the problem (in
this case, the number of grams of propane). Don't forget to include
the formula for the compound you're starting with:
-
Put a multiplication
sign after the number of moles and draw a line. Above and below the
line, write the molar mass of propane.
Write it so that "g C3H8" cancel out
leaving you with mol C3H8.
| g CO2 |
= |
15.0 g C3H8 |
x |
1 mol C3H8 |
| |
|
|
|
44.0 g C3H8 |
-
Cancel out your units
and compare them to the ones you want to end up with. Since
they are not the same, multiply by the molar ratio. Remember
to write the molar ratio so that "mol C3H8"
cancels out. Use the coefficients from your balanced chemical equation
to complete your molar ratio.
| g CO2 |
= |
15.0 g C3H8 |
x |
1 mol C3H8 |
x |
3 mol CO2 |
| |
|
|
|
44.0 g C3H8 |
|
1 mol C3H8 |
-
Cancel out your units
and compare the ones that are left to the ones you want. Since
they are not the same, multiply by the molar mass of carbon
dioxide. Remember to write the molar mass as
a conversion factor so that "mol CO2"
cancels out leaving you with "g CO2"
g CO2
|
=
|
15.0 g C3H8
|
x
|
1 mol C3H8
|
x
|
3 mol CO2
|
x
|
44.0 g CO2
|
|
|
|
|
|
44.0 g C3H8
|
|
1 mol C3H8
|
|
1 mol CO2
|
-
Cancel out your units
and compare the ones that are left to the ones you want. Since
they are the same, do the math. Report
your answer using the correct units and the correct number of signficant
figures.
g CO2
|
=
|
15.0 g C3H8
|
x
|
1 mol C3H8
|
x
|
3 mol CO2
|
x
|
44.0 g CO2
|
|
|
|
|
|
44.0 g C3H8
|
|
1 mol C3H8
|
|
1 mol CO2
|
g CO2
|
=
|
45.0 g CO2
|
|
|
|
|
|
|
NOTE:
By now you've noticed that the molar masses of propane and carbon
dioxide are identical. This is NOT very common. In most cases, your
reactants and products all have different molar masses.
|
|
|
|
|
|
|
|
Practice Problems
|
|
|
|
|
|
|
|
Use the following balanced equation to solve
the problems below:
Fe2O3
(s) + 6 HCl (g) --> 2 FeCl3 (s) + 3 H2O (g)
- How many grams of FeCl3 can
be produced using 0.295 mol Fe2O3?
- How many grams of HCl are needed to react
with 27.62 g of Fe2O3?
- How many moles of HCl are needed to produce
15.5 g of FeCl3?
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|