| A | B |
|---|
| What is the last name of the scientist who first suggested that electrons can be thought of as waves? | de Broglie |
| What is the last name of the scientist who first suggested that it is impossible to know both the position and the velocity of an electron at the same time? | Heisenberg |
| Louise de Broglie was the first to suggest that electrons can be thought of as both a particle and a ______. | wave |
| The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the _________ and __________ of an electron or any other particle. | positiion, velocity |
| What is the last name of the scientist who developed an equation that treated electrons as waves? | Schrodinger |
| _______ theory describes mathematically the wave properties of electrons and other very small particles. | Quantum |
| According to quantum theory, electrons do not travel around the nucleus in neat orbits, but instead exist in certain regions called ______. | orbitals |
| What are the letters that describe the first four shapes of orbitals? | s, p, d, f |
| What is the angular momentum quantum number of an s-shaped orbital? | l = 0 (That's a letter L before the = sign) |
| An orbital is a three-dimensional region around the nucleus that indicates the ___________ location of an electron. | probable |
| What is the angular momentum quantum number of a p-shaped orbital? | l = 1 (That's a letter L before the = sign) |
| What is the angular momentum quantum number of a d-shaped orbital? | l = 2 (That's a letter L before the = sign) |
| What is the angular momentum quantum number of an f-shaped orbital? | l = 3 (That's a letter L before the = sign) |
| l = 3 describes a(n) ______. (That's a letter L before the = sign) | f-shaped orbital |
| What makes an electron spin in either the positive or negative direction? | A Chuck Norris spinning round house kick depending on whether it's off the left or right foot |
| What is the maximum number of p-orbitals in an energy level? | 3, 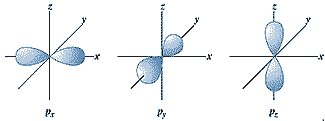 |
| What is the maximum number of d-orbitals in an energy level? | 5,  |
| The magnetic quantum number, symbolized by m, indicates the ___________ of an orbital around the nucleus. | orientation |
| The ________ quantum number indicates the orientation of an orbital around the nucleus. | magnetic quantum number |
Elements in this part of the periodic table have their highest energy electrons in ___ shaped orbitals., 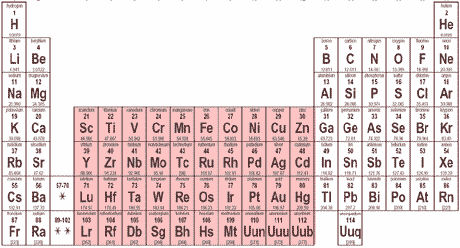 | d,  |
Elements in this part of the periodic table have their highest energy electrons in ___ shaped orbitals.,  | f,  |
Elements in this part of the periodic table have their highest energy electrons in ___ shaped orbitals.,  | p,  |
What is the shape of the orbital shown below?,  | p-shaped,  |
| The ________ quantum number indicates the shape of an orbital around the nucleus. | angular momentum quantum number, l (<-- an L) |
| The angular momentum quantum number, symbolized by the letter "l" , indicates the _____ of an orbital around the nucleus. | shape |
Elements in this part of the periodic table have their highest energy electrons in ___ shaped orbitals.,  | s,  |
What is the shape of the orbital shown below?, 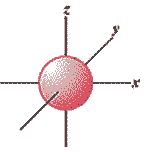 | s-shaped,  |
What is the shape of the orbital shown below?, 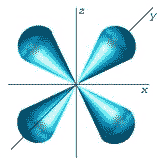 | d-shaped,  |
| If an orbital has the maximum of 2 electrons, one must have a spin of ____ and the other must have a spin of ____. | +1/2, -1/2 |
| The ________ quantum number, indicates the spin of an electron. | spin quantum number |
| Which type of quantum number can only have values of +1/2 or -1/2? | spin quantum number |
| The _______ quantum number, symbolized by the letter n , indicates the main energy level occupied by the electron. | principal |
| What is the principal quantum number of the lowest possible energy level? | n=1 |
| The second electron shell would have a principle quantum number of _____. | n=2 |
| Elements with 3 energy levels (a.k.a. electron shells) would be found in the ____ period. | 3rd |
| Because the first energy level can have only one s-orbital and no other types of orbitals, the first energy level can hold a maximum of ___ electrons. | 2 |
| Because the second energy level can have only have one s-orbital and 3 p-orbitals, the second energy level can hold a maximum of ____ electrons. | 8 |
| What is the maximum number of f-orbitals in an energy level? | 7 |
| For any orbital, the maximum number of electrons it can hold is ___. | 2 |
What is the principal quantum number of the outermost energy level in this diagram?, 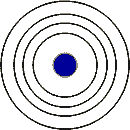 | n=4,  |
Elements with the same number of electron shells as in the picture below can be found in the ___ period.,  | fourth,  |