 |
Java Games: Flashcards, matching, concentration, and word search. |
 |
 |
GENERAL CHEMISTRY - The Electromagnetic Spectrum
|
| A | B |
|---|
According to the chart below, visible light has a wavelength range between ______ at the violet end and _____ at the red end.,  | 380 nanometers (violet end), 750 nm at the red end,  |
Violet light has ____ energy, _____ wavelength and ______ frequency compared to red light.,  | violet light has greater energy, shorter wavelength and higher frequency compared to red light,  |
| When electromagnetic energy hits matter, it can either be _____, _____, or _____ depending on the wavelength of energy and type of matter. | absorbed, reflected, or transmitted |
| When electromagnetic energy hits matter, it can either be absorbed, reflected, or transmitted depending on the _______ and _____. | wavelength of energy and the type of matter |
The red arrow signifies ______., 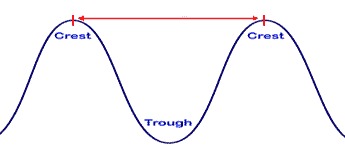 | The wavelength of this wave,  |
Which type of electromagnetic radiation has the highest energy?,  | gamma rays,  |
Which type of electromagnetic radiation has the highest frequency?,  | gamma rays,  |
Which type of electromagnetic radiation has the longest wavelength?,  | radiowaves,  |
Which type of electromagnetic radiation has the shortest wavelength?,  | gamma rays,  |
Which type of electromagnetic radiation has the lowest frequency?,  | radiowaves,  |
Which type of electromagnetic radiation has the lowest energy?,  | radiowaves,  |
The type of electromagnetic radiation that you would find having a wavelength slightly shorter than violet light would be _______.,  | ultra violet (UV) radiation,  |
The type of electromagnetic radiation that you would find having a wavelength slightly longer than red light would be _______.,  | infrared,  |
Infrared radiation has a slightly ______ wavelength than red light.,  | longer,  |
Ultra violet (UV) radiation has a slightly ______ wavelength than violet light.,  | shorter,  |
The frequency of electromagnetic waves is _______ proportional to the energy of those waves.,  | directly,  |
The energy of electromagnetic waves is _______ proportional to the frequency of those waves.,  | directly,  |
The higher the frequency of waves, the ______ the energy of those waves.,  | higher,  |
The longer the wavelength of a wave, the _____ the frequency of those waves.,  | lower,  |
The longer the wavelength of a wave, the _____ the energy of those waves.,  | lower,  |
| What is smaller than an electron? | Chuck Norris' sense of fear (but no one has been brave enough to discover it yet) |
|
 |
 |
|
|
|
| |