| A | B |
|---|
What does this show?, 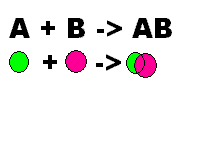 | This shows synthesis |
What does this show?, 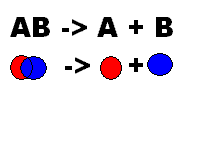 | This shows decomposition |
What does this show?, 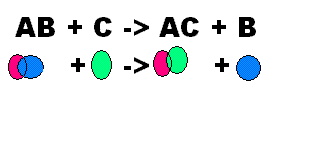 | This shows a Single Replacement |
What does this show?, 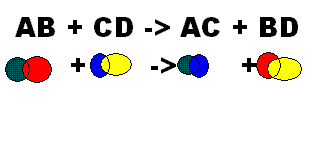 | Double Replacement |
| Occurs when different atoms in two different compounds trade places | This shows a Double Replacement |
| Compounds break down into simpler substances | Decomposition |
| Occurs when one element replaces another one in a compound | Single Replacement |
| Two or more elements or compounds combine to make a more complex substance | Synthesis |
| Where do you find the reactants for a chemical equation? | On the left (before arrow) |
| Where do you find the products for a chemical reaction? | On the right (after arrow) |
| What are some signs of a chemical reaction? | bubbling, color change, temperature change |
| How can you speed up a chemical reaction? | increase heat, make pieces of a solid smaller |
| What does the Law of Conservation of Matter state? | Matter cannot be created or destroyed in a reaction, only changed. |