| A | B |
|---|
The highlighted portion of the periodic table is called the ___ block., 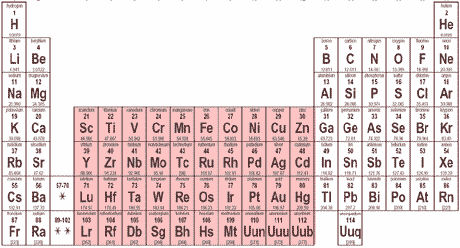 | d (This is where all of the transition metals are located),  |
The highlighted portion of the periodic table is called the ___ block.,  | f,  |
The highlighted portion of the periodic table is called the ___ block.,  | p,  |
The highlighted portion of the periodic table is called the ___ block.,  | s,  |
| Elements with 3 electron shells would be found in the ____ period. | 3rd |
Elements with the same number of electron shells as in the picture below can be found in the ___ period., 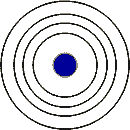 | fourth,  |
| Who created the first periodic table in 1869? | Mendeleev |
| Repeating properties are also called ____ properties. | periodic |
| Boron,silicon,germanium, arsenic, antimony, and tellerium are ________. | metalloids, 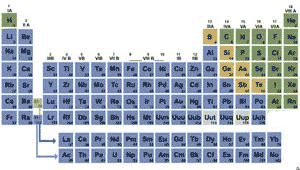 |
The elements highlighted in green are classified as ____.,  | non-metals,  |
The elements highlighted in yellow are classified as ____.,  | metalloids,  |
| The elements highlighted in blue are classified as ____. | metals |
| Electrons available to be lost, gained, or shared ina chemical reactions are called ______. | valence electrons |
| Electrons in the outermost shell of an atom are called _____. | valence electrons |
| Where are valence electrons located? | In the outermost shell of the atom. |
| The ______ states that atoms want to gain, lose, or share electrons to get a total of eight electrons in their outermost shell. | octet rule |
| The octet rule states that atoms want to gain, lose, or share electrons to get a total of _____ electrons in their outermost shell. | eight |
| The octet rule states that atoms want to gain, lose, or share electrons to get a total of eight electrons in their _____ | outermost shell |
| Atoms with eight electrons in their outermost shell are considered to be ____ or ____. | stable, unreactive |
| Atoms with full outer shells are in the _____ family. | noble gas |
| What's the only noble gas to violate the octet rule? | helium (because it only has one electron shell and it fills that shell with its two electrons) |
| Group one elements have ______ valence electron. In each successive group as you move across groups in the S and P blocks, the number of valence electrons increases by ____. | 1,1 |
| The charge that chemically combined or ionized atoms have acquired by gaining or losing electrons to get an octet of electrons describes the ______ of the element. | oxidation number |
| The oxidation number of group I elements is ____. | +1 |
| The oxidation number of group II elements is ____. | +2 |
The oxidation number of the group that includes flourine, chlorine, and bromine is _____.,  | -1,  |
What is the oxidation number of the noble gas family?,  | 0,  |
The oxidation number of the group that includes oxygen and sulfur is _____.,  | -2,  |
The most common oxidation number of the group that includes carbon and silicon is _____.,  | +4 or -4,  |
| As you go down a group of elements on the periodic table, the atomic radius _____. | increases |
| As you across a group of elements on the periodic table from left to right, the atomic radius _____. | decreases |
| As you go down a group of elements on the periodic table, the elements' electronegativity _____. | decreases |
| As you across a group of elements on the periodic table from left to right, the elements' electronegativity _____. | increases |
| As you across a group of elements on the periodic table from left to right, the atomic radius decreases due to the increasing number of ______. | protons in the nucleus (positive protons attract the negatively charged electron cloud in closer) |
| As you go down a group of elements on the periodic table, the atomic radius increases due to the increasing number of _____ that each element in a group gains. | electron shells |
| ______ is a measure of how strongly an atom attracts electrons from other atoms. | Electronegativity |
| Electronegativity is a measure of how strongly an atom attracts _______ from other atoms. | electrons |
Group 1 elements (shown in red) are know as the ________,  | alkali metals,  |
Group 2 elements (shown in orange) are know as the ________,  | alkaline earth metals,  |
Group 17 elements (shown in dark blue) are know as the _________,  | halogens,  |
Group 18 elements (shown in purple) are know as the _________,  | noble gases,  |
Elements in groups 3 -12 (shown in grey) are known as the _______.,  | transition metals,  |