| A | B |
|---|
| Molecules are held together by _____ bonds. | covalent |
| A(n) ______ is held together by covalent bonds. | molecule |
| Two formulas can be used to describe hydrogen peroxide. HO, and H2O2. Which one is the molecular formula and which is the empirical formula? What's the difference between these types of formulas? | HO is the empirical formula. Empirical formulas show the lowest common ratio of elements in a compound while molecular formulas indicate the actual number of atoms of each type in a molecule. |
| Diatomic molecules consist of ____ | 2 atoms bonded together. |
| Which elements are found in diatomic form in nature? | hydrogen, nitrogen, oxygen, flourine, chlorine, bromine and iodine (remember the heavenly 7. See periodic table) |
| Polar molecules have a ______ end and a ______ end. | partial positive, partial negative |
| ______ molecules have a partial positive end and a partial negative end. | polar |
| _____ molecules can't be divided into poles of opposite charge. | non-polar |
| Organic molecules contain _____ and _____ and were originally made inside living things. | carbon, hydrogen |
| _______ molecules contain carbon and hydrogen and were originally made inside living things. | Organic |
| _______ molecules contain carbon and hydrogen and were originally made inside _______. | organic, living things |
| Energy is ______ when bonds form. | released |
| Energy is required to ___________ | break a chemical bond |
| Chemical compounds tend to form so that each atom, by _________, ________, or _________ electrons, has an ________ of electrons in its highest energy level. | gaining, losing, or sharing electrons, octet |
| Which three atoms are exceptions to the octet rule and how many electrons do they tend to have in their outer shell when they form compounds? | Hydrogen (2), Beryllium (4), Boron (6) |
| Electron dot notation uses dots around the element's symbol to show the element's ______. | outer shell electrons |
| Lewis structures are like electron dot notation, except they show _______ instead of _______. | molecules, elements |
| Electrons in the outer electron shell that are not involved with bonding are called ______. | lone pair electrons |
| The structural formula shows ______. | Just the symbols of the elements in a compound with dashes to symbolize bonds (The one in the middle is a structural formula. The other two are Lewis structures), 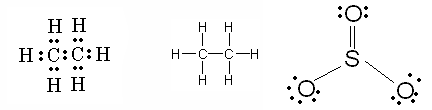 |
| How would you show the formation of a covalent compound using electron dot notation and Lewis structures? Use the formation of bromine as an example. | .,  |
| Ionic compounds are made of individual _____. | ions |
| Draw the formation of the ionic compound NaCl from a sodium atom and a chlorine atom. | .,  |
| A charged group of covalently bonded atoms is called a(n) ___________. | polyatomic ion (Examples include |
| Which of the following molecules are diatomic? C-O (carbon monoxide) or 0=0 (elemental oxygen, O2) | They both are since they both contain exactly 2 atoms. |
Which of these diagrams is a structural formula and which is a Lewis structure?,  | The one in the middle is a structural formula and the outer ones are Lewis structures (Notice that structural formulas show no dots, just bonds represented by dashes),  |
| Positively charged ions are called ______. | cations |
| Negatively charged ions are called ______. | anions |