| A | B |
|---|
| Bond Energy | Is the strength of a chemical bond between atoms, expressed as the amount Of energy required to break it apart., 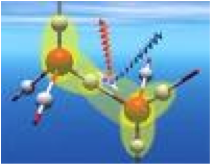 |
| Chemical Formula | A chemical formula enumerates the elements in a molecule.,  |
| Covalent Bond | A covalent bond is a chemical link between two atoms in which electrons are shared between them., 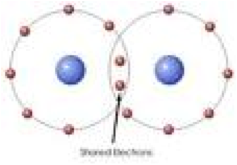 |
| Covalent Bonding | Bond that is formed when two atoms share electrons,  |
| Dipole | A pair of electric point charges or magnetic poles of equal magnitude and opposite signs, separated by an infinitesimal distance., 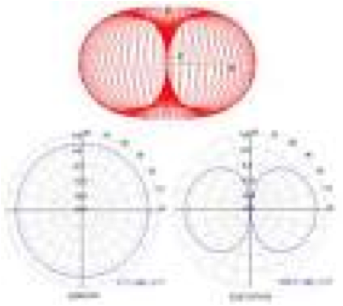 |
| Dispersion Forces | Also known as London dispersion forces. Dispersion is an intermolecular attraction force that exists between all molecules. These forces are the result of the movement of electrons, which cause slight polar moments. Dispersion forces are generally very weak but as the molecular weight increases so does the strength., 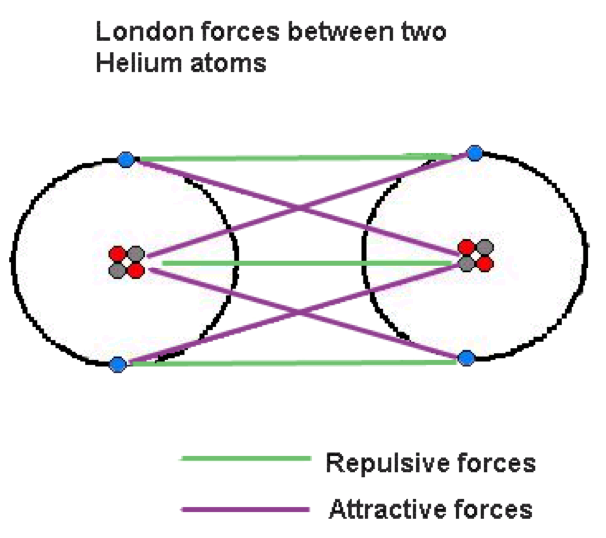 |
| Ductility | The property of a metal that enables it to stretch before rupturing., 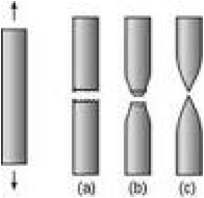 |
| Electron Dot Notation | This notation use dots placed around the symbol of the element to represent the valence,  |
| Formula Unit | The chemical formula with the least number of elements out of the set of empirical formulas having the same proportion of ions as elements,  |
| Hybrid Orbitals | An orbital formed by the combination of two or more atomic orbitals on a single., 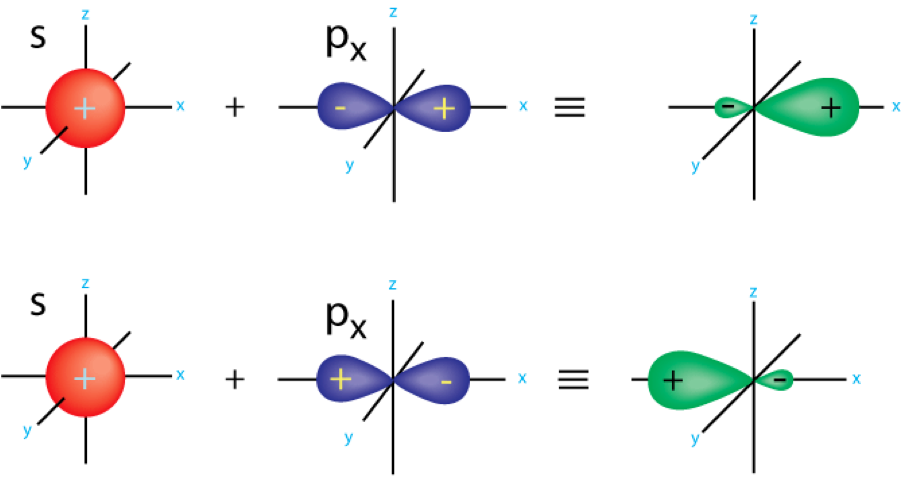 |
| Hybridization | Mixing a set of atomic orbital’s to form a new set of atomic orbital’s with the same total electron capacity and properties and energies intermediate between those of the original unhybridized orbital., 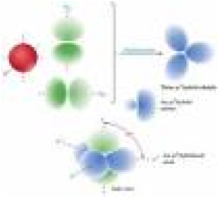 |
| Hydrogen Bonding | Is the intermolecular force most often associated with water. It is a weak type of bond that occurs when a hydrogen atom bonds with a highly electronegative atom like oxygen., 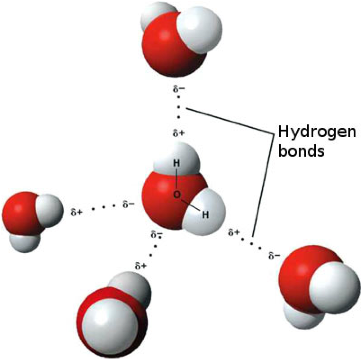 |
| Ionic Bonding | A chemical link between two atoms caused by the electrostatic force between oppositely-charged ions in an ionic compound., 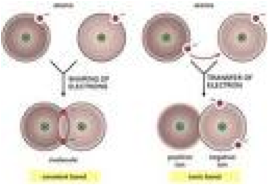 |
| Ionic Compound | A chemical compound of cations and anions, which are held together by ionic bonds in a lattice structure, 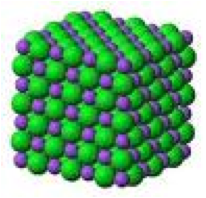 |
| Malleability | The property of being physically malleable; the property of something that can be worked or hammered or shaped without breaking.,  |
| Metallic Bonding | The electromagnetic interaction between delocalized electrons, called conduction electrons, gathered in an "electron sea", and the metallic nuclei within metals., 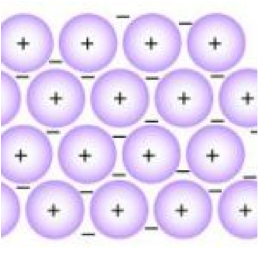 |
| Molecular Compound | A compound that is composed of molecules.,  |
| Molecular Formula | A formula in which only the elements and number of atoms of each element are shown., 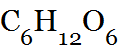 |
| Molecule | The simplest structural unit of an element or compound,  |
| Non-Polar | A type of covalent bond in which both atoms attract the bonding electrons, 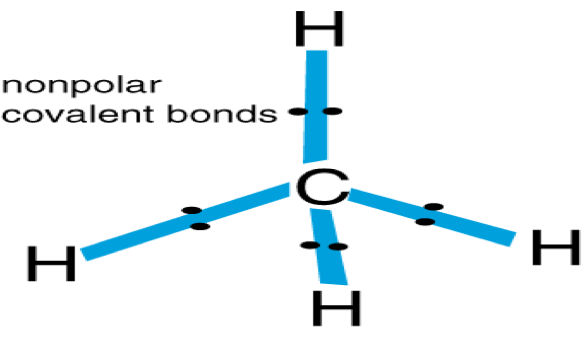 |
| Polar | Occurs when one of the elements attracts the shared electrons more strongly than the other element.,  |
| Polar Covalent Bond | The electrons shared by the atoms spend a greater amount of time, on the average, closer to the Oxygen nucleus than the Hydrogen nucleus., 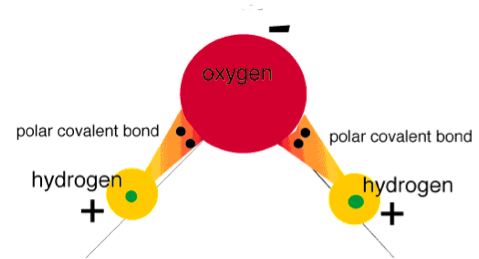 |
| Polyatomic Ion | A charged particle which has two or more atoms held together by covalent bonds.,  |
| VSEPR Theory | Valence shell electron pair repulsion (VSEPR) theory is a model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion, 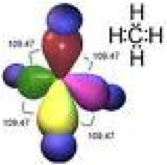 |
| Chemical Bond | An electrical force linking atoms,  |
| Lewis Structure | Diagrams that show the bonding between atoms of a molecule, 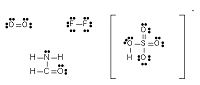 |
| Structural Formula | An expanded molecular formula showing the arrangement of atoms within the molecule, 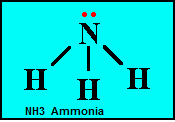 |
| Single Bond | A covalent bond in which one electron pair is shared between two atoms,  |
| Multiple Bond | Sharing of more than one electron pair between bonded atoms, 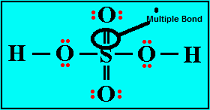 |
| Resonance | Resonance is the tendency of a system (usually a linear system) to oscillate at larger amplitude at some frequencies than at others,  |
| London Dispersion Forces | Attractive or repulsive force between molecules,  |