| A | B |
|---|
| All matter is made up of roughly 100 different types of particle called... | Atoms |
| Materials which are made up of only one type of atom are called... | Elements |
| An electrically neutral group of more than one atom held together by covalent bonds | Molecule |
| A particle which has an unequal number of protons and electrons, giving it an electrical charge. | Ion |
| Two or more different atoms held together by covalent bonds | Compound |
| A group of elements which are all malleable, lustrous, sonorous, ductile and good conductors of heat and electricity. | Metals |
| A group of elements which are generally dull and brittle, poor conductors of heat and electricity and form acidic oxides | Non-metals |
| More than one type of particle jumbled up together but not bonded. Can be easily separated using physical methods | Mixture |
| Ions are formed by chemical reactions which cause | Electron Transfer |
| Molecules and compounds are formed when atoms | Share Electrons |
| Metallic bonds are caused by _________ electrons | Delocalised |
| Polar compounds are formed when electrons are shared | Unevenly |
Name the molecule shape, 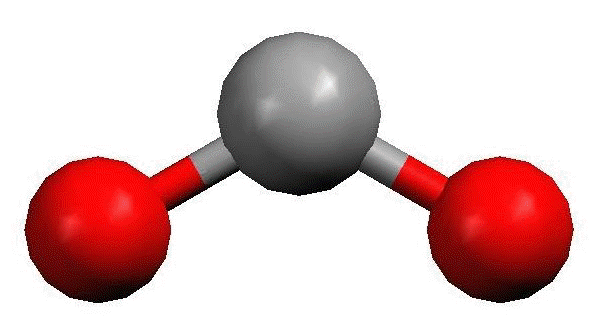 | Bent |
Name the molecule shape, 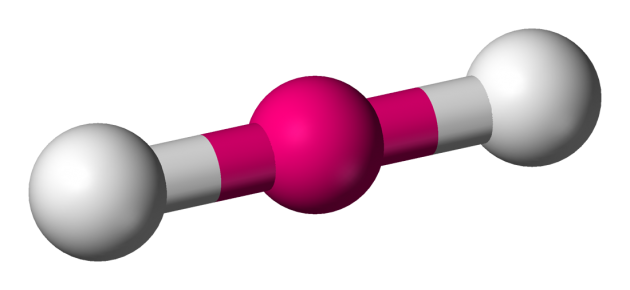 | Linear |
Name the molecule shape, 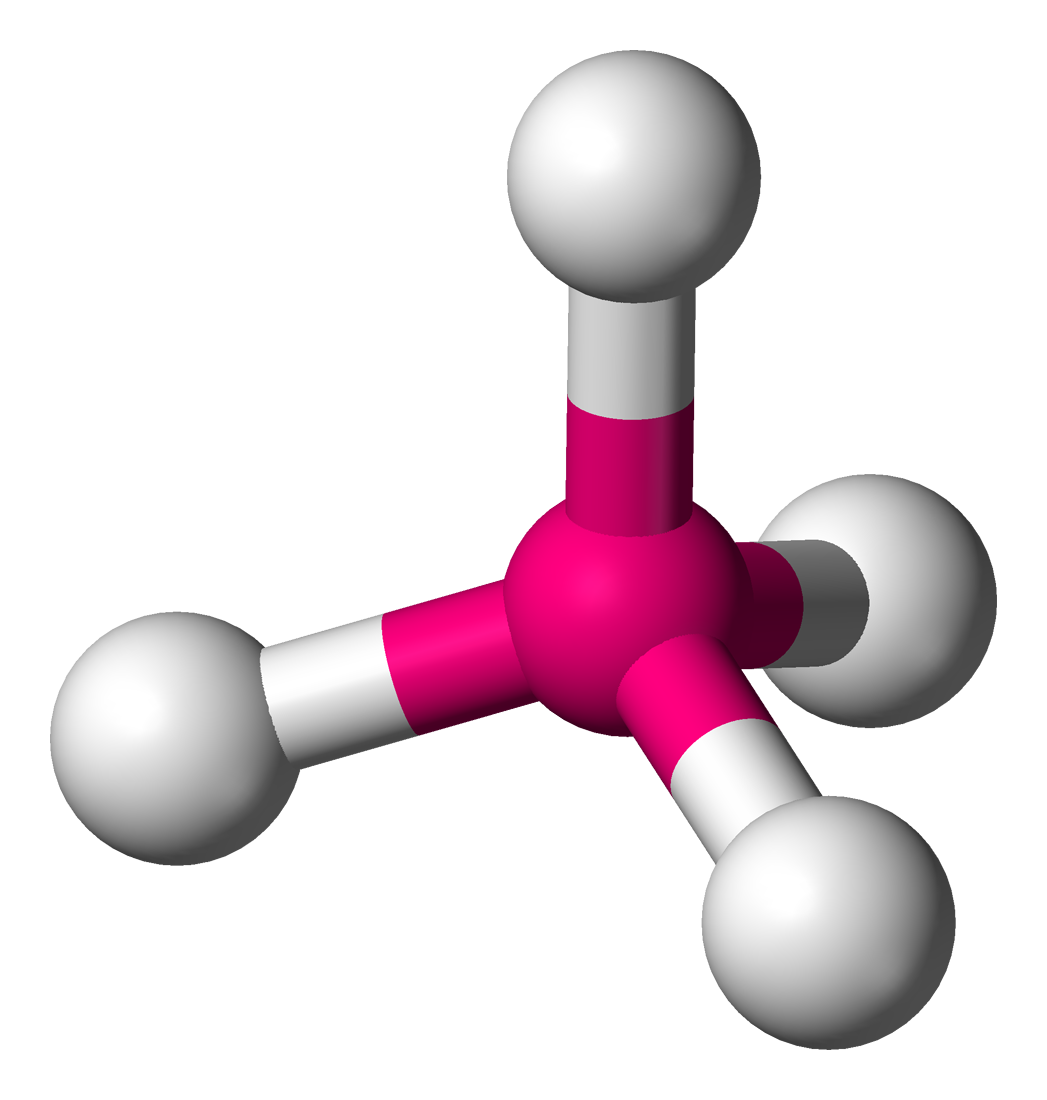 | Tetrahedral |
Name the molecule shape, 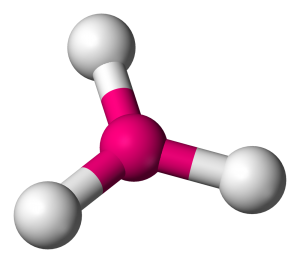 | Trigonal Planar |
Name the molecule shape, 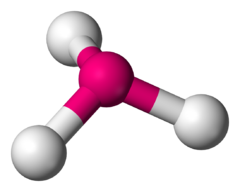 | Trigonal Pyramid |