| A | B |
|---|
| Aliphatic hydrocarbons | Prolonged exposure and inhalation may cause irritation to skin, digestive system, throat, and lungs, Neurotoxic |
| Alkanes | Methane series: a series of non-aromatic saturated hydrocarbons with the general formula CnH(2n+2), 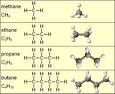 |
| Alkene | An alkene is a hydrocarbon containing a double carbon-carbon bond., 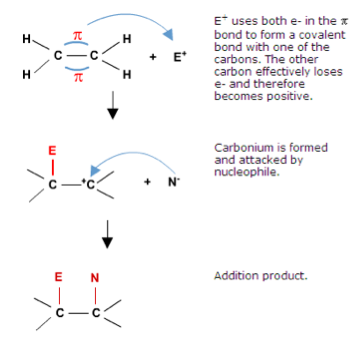 |
| Alkyl Group | Any of a series of univalent groups of the general formula CnH2n+1 derived from aliphatic hydrocarbons, 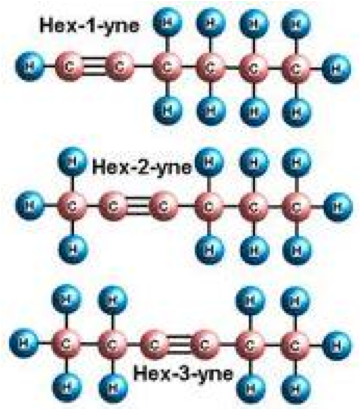 |
| Alkynes | Are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2,  |
| Aromatic Compound | Also known as arenes or aromatics, are chemical compounds that contain conjugated planar ring systems with delocalized pi electron clouds instead of discrete alternating single and double bonds.,  |
| Asymmetric carbon | An aldohexose with 4 asymmetric carbon atoms has 24 = 16 stereoisomers: The four groups of atoms attached to the carbon atom can be arranged in space in two different ways that are mirror images of each other, and which lead to so-called left-handed and right-handed versions of the same molecule.,  |
| Branched-chain alkane | Any of its carbon atoms is joined to more than two other carbon atoms, 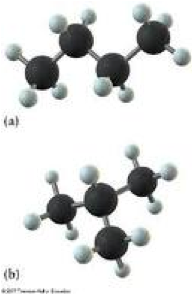 |
| cis configuration | cis-trans isomerism or geometric isomerism or configuration isomerism or E-Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule, 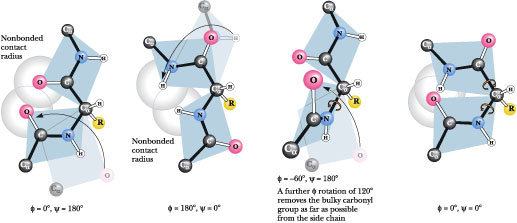 |
| Condensed Structural Formulas | A structural representation of a compound that includes all of the atoms present in a molecule or other chemical entity but represents only certain bonds as lines in order to emphasize a structural characteristic., 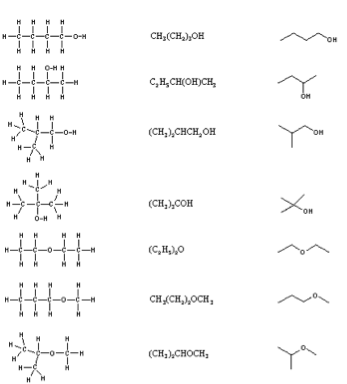 |
| Crackling | Fifth derivative of the position vector with respect to time,  |
| Cyclic hydrocarbon | An organic compound that contains carbon and hydrogen only., 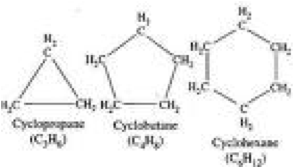 |
| Geometric isomers | A chemical species with the same type and quantity of atoms as another species, yet having a different geometric structure., 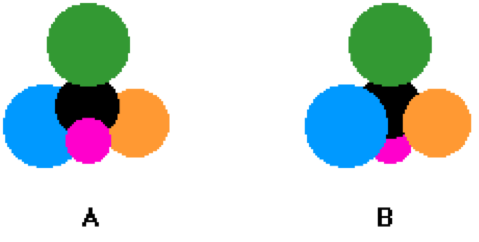 |
| Homologous series | A series is a series of organic compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group, and shows a gradation in physical properties as a result of increase in molecular size and mass,  |
| Hydrocarbons | An organic compound consisting entirely of hydrogen and carbon, 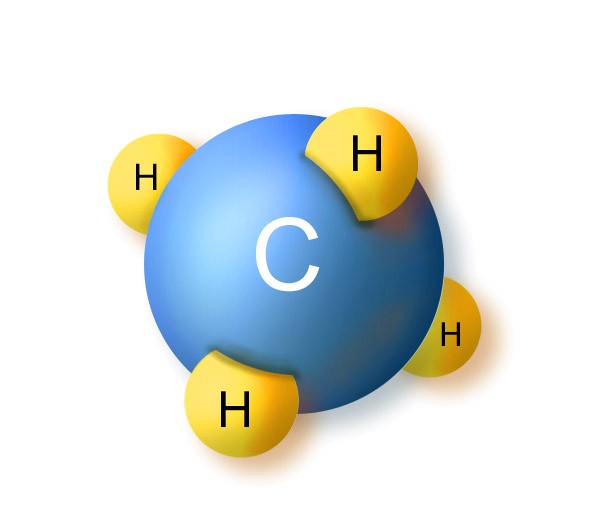 |
| Isomers | A chemical species with the same number and types of atoms as another chemical species, but possessing different properties. There are structural isomers, geometric isomers, optical isomers, and stereoisomers.,  |
| Optical isomers | A chiral molecule is a type of molecule that lacks an internal plane of symmetry and has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom., 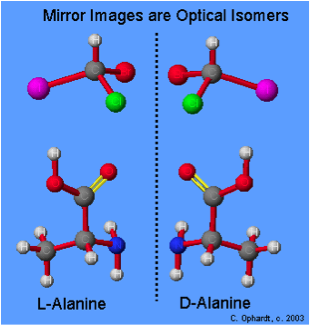 |
| saturated compounds | Compounds in which carbon atoms are joined together by single bonds,  |
| Stereoisomers | One of a set of isomers whose molecules have the same atoms bonded to each other but differ in the way these atoms are arranged in space., 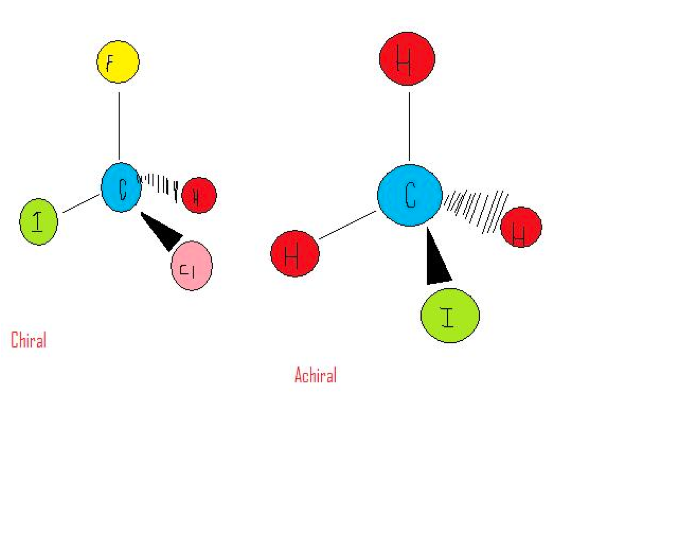 |
| Straight chain alkanes | One of a set of isomers whose molecules have the same atoms bonded to each other but differ in the way these atoms are arranged in space., 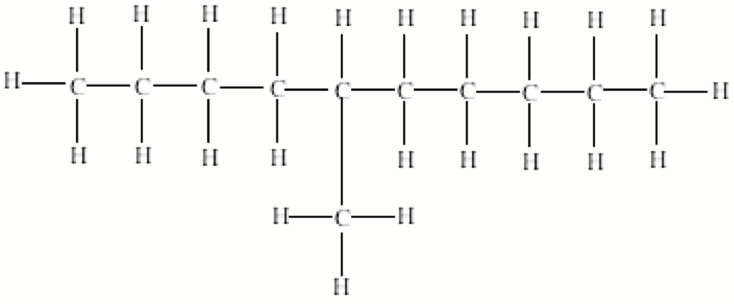 |
| Structural isomers | Constitutional isomerism in accordance with IUPAC, is a form of isomerism in which molecules with the same molecular formula have atoms bonded together in different orders, as opposed to stereoisomerism., 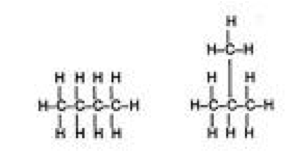 |
| Substinent | Is an atom or group of atoms substituted in place of a hydrogen,  |
| Trans configuration | In organic chemistry, cis-trans isomerism or geometric isomerism or configuration isomerism or E-Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule., 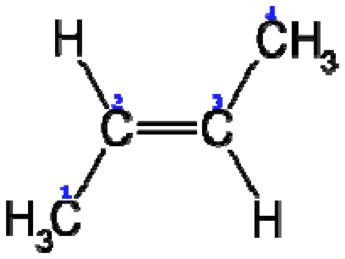 |
| Unsaturated compound | Is a chemical compound that contains carbon-carbon double bonds or triple bonds such as in alkenes or alkynes. In a saturated compound these double bonds are removed by the addition of hydrogen and no multiple bonds are present, 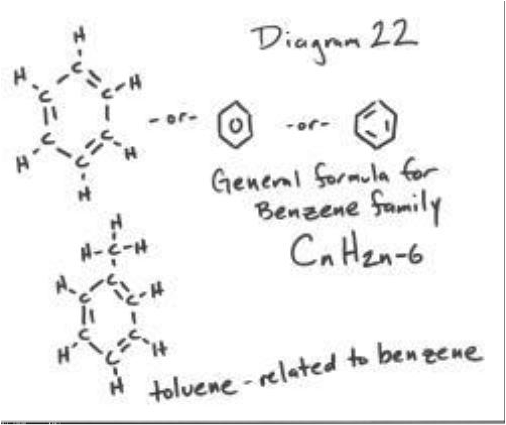 |