| A | B |
|---|
| Standard Temperature | Exactly zero degrees centigrade,  |
| Pressure | The force applied to a unit area of surface; measured in pascals (SI unit), 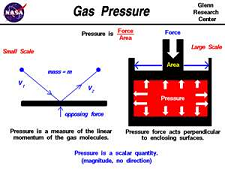 |
| Mole | Is the amount of pure substance containing the same number of chemical units as there are atoms in exactly 12 grams of carbon-12.,  |
| Avogadro's Hypothesis | The principle that equal volumes of all gases (given the same temperature and pressure) contain equal numbers of molecules,  |
| Avogadro's Number | The number of particles found in one mole of a substance. It is the number of atoms in exactly 12 grams of carbon-12. Is approximately 6.022 x 10^23 particles per mole.,  |
| Empirical Formula | A chemical formula showing the ratio of elements in a compound rather than the total number of atoms?, 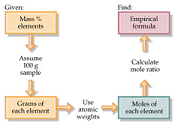 |
| Molar Mass | The mass of one mole of a substance, usually expressed in grams or kilograms.,  |
| Molar Volume | The molar volume, symbol Vm, is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure., 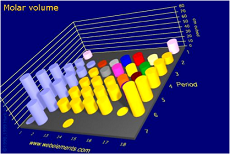 |
| Percent Composition | The percent composition (percentage composition) of a compound is a relative measure of the mass of each different element present in the compound., 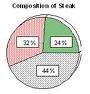 |
| Representative Particle | The smallest unit into which a substance may be broken down without a change in composition.,  |