| A | B |
|---|
| Chemical Change | Is a change that produces matter with a different composition than the original matter.,  |
| Chemical Property | The ability of a substance to undergo a specific chemical change is called a chemical property. |
| Chemical Reaction | A process that changes the molecular composition of a substance by redistributing atoms or groups of atoms without altering the structure of the nuclei of the atoms,  |
| Chemical Symbol | Each element is represented by a one-or two letter symbol,  |
| Compound | Is a substance that contains two or more elements chemically combined in a fixed proportion |
| Distillation | Liquid is boiled to produce a vapor that is then condensed into a liquid, 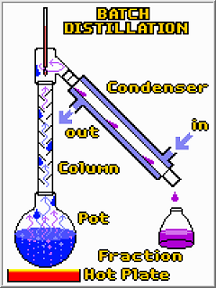 |
| Element | Is the simplist form of matter that has a unique set of properties.,  |
| Extensive Properties | Is a property that depends on the amount of matter in a substance., 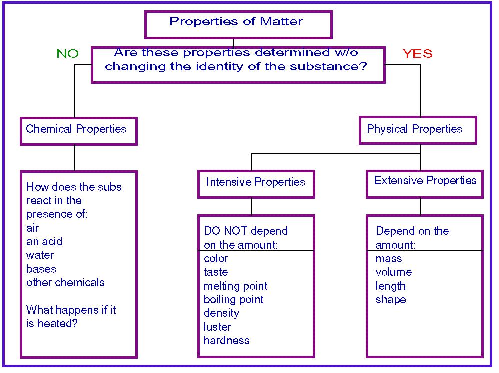 |
| Filtration | The process that seperates a solid from the liquid in a heterogenous mixture is called filtration., 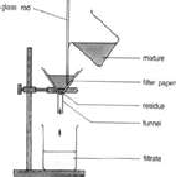 |
| Gas | Form of matter that takes up both the space and the volume of it's container, 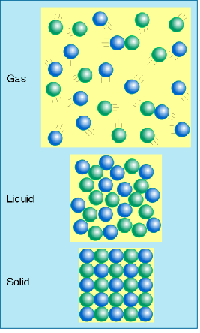 |
| Heterogeneous Mixture | A mixture in which the composition is not uniform through out is a heterogeneous mixture, 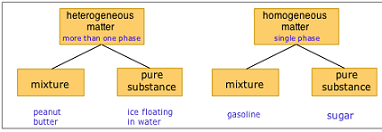 |
| Homogeneouse Mixture | Is a mixture in which the compoition is uniformthroughut.,  |
| Intensive Property | Substance that depends on the type of matter in a sample, not the amount of matter.,  |
| Law of Conservation of Mass | States that in any phyical change or chemica reaction, mass is conserved., 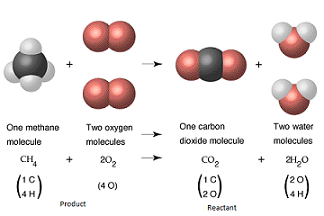 |
| Liquid | It is a form of matter hat has an indefinite shape flows yet has a fixed volume., 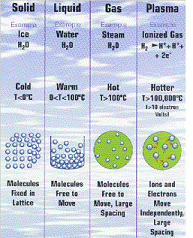 |
| Mass | Measure of the amount of matter an object contains. |
| Mixture | Physical blend of two or more components., 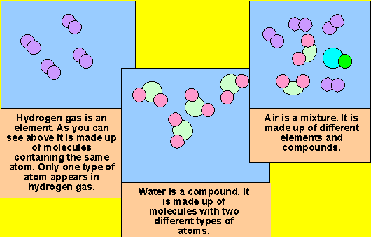 |
| Phase | Describes any part o a sample with uniform composition and properties. |
| Physical Change | Some properties of material change but the composition of the material does not.,  |
| Physical Property | Is a quality or condition of a substance that can be obsorved or measure with out changing the substance composition. |
| Precipitate | A solid that forms and settles out of a liquid mixture.,  |
| Product | Substance produced in a reactant,  |
| Reactant | A substance present n the beginning of a reaction,  |
| Solid | Is a form of matter that has a definite shape and volume., 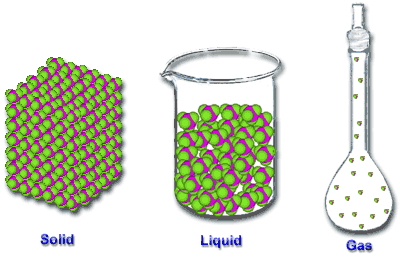 |
| Solution | A homogeneous mixture; Consists of solutes disolved in a solvent.,  |
| Substance | Matter that has a uniform and definite compositon.,  |
| Vapor | Describes the gaseous state of a substance that is generally a liquid or solid at room temperature,  |
| Volume | A measure of the space occupied by a sample of matter. |