 |
Java Games: Flashcards, matching, concentration, and word search. |
 |
 |
Chap 12 - Stoichiometry
Key terms to know to be successful in this chapter.
|
| A | B |
|---|
| Actual Yield | The quantity of a product that is obtained from a chemical reaction (as opposed to the calculated or theoretical yield).,  |
| Excess Reagent | Reactants that are not used up when the reaction is finished, 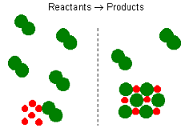 |
| Limiting Reagent | Also known as the "limiting reactant", is the chemical that determines how far the reaction will go before the chemical in question gets "used up", causing the reaction to stop.,  |
| Mole Ratio | A fractional, comparison of mole amounts of atoms, molecules, and formula units. Just as the number of atoms in a molecule or formula unit can be counted, so can the number of moles of atoms in moles of molecules be counted. Thus, the formula C3H6O3 can have the atom-molecule and mol-mol ratios., 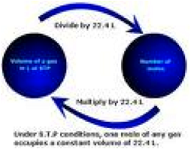 |
| Percent Yield | Is calculated to be the experimental yield divided by theoretical yield multiplied by 100%.,  |
| Stoichiometry | Is the study of relationships or ratios between two or more substances undergoing a physical or chemical change.,  |
| Theoretical Yield | Is the amount of product obtained in a chemical reaction,  |
| Percent Yield Equation | Use this equation calculate percent yield., 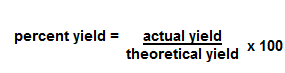 |
| Mole to Mole Relationship Equation | Use this equation to convert from moles of one element to moles of another element., 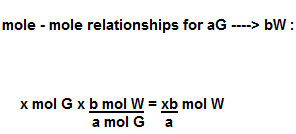 |
|
 |
 |
|
|
|
| |