| A | B |
|---|
| Strong Electrolyte | Is a solute that completely, or almost completely, ionizes or dissociates in a solution.,  |
| Aqueous Solution | Any solution in which water is the solvent., 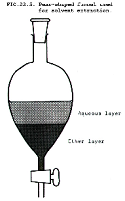 |
| Brownian Motion | The random movement of microscopic particles suspended in a liquid or gas, caused by collisions with molecules of the surrounding.,  |
| Colloid | A substance that consists of particles dispersed throughout another substance which are too small for resolution with an ordinary light microscope but are incapable of passing through a semipermeable membrane,  |
| Electrolyte | A melted or dissolved compound that has broken apart into ions (anions and cations). Applying an electric field across an electrolyte causes the anions and cations to move in opposite directions, thereby conducting electrical current while gradually separating the ions. See also electrodialysis, electrolysis.,  |
| Emulsion | A suspension of small globules of one liquid in a second liquid with which the first will not mix,  |
| Hydrate | A solid compound containing water molecules combined in a definite ratio as an integral part of the crystal,  |
| Nonelectrolyte | A substance that does exist in an ionic form in aqueous solution., 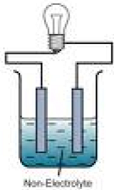 |
| Solute | The substance that is dissolved in a solution.,  |
| Solvation | Any of a class of chemical reactions, such as the formation of hydrated copper sulfate in aqueous solution, in which solute and solvent molecules combine with relatively weak covalent bonds.,  |
| Solvent | A substance in which another substance is dissolved, forming a solution.,  |
| Surface Tension | The elastic like force existing in the surface of a body, esp. a liquid, tending to minimize the area of the surface, caused by asymmetries in the intermolecular forces between surface molecules.,  |
| Surfactant | Is a complex substance containing phospholipids and a number of a proteins,  |
| Suspension | A system consisting of small particles kept dispersed by agitation (mechanical suspension) or by the molecular motion in the surrounding medium (colloidal suspension).,  |
| Tyndall Effect | Is light scattering by particles in a colloid or particles in a fine suspension,  |
| Weak Electrolyte | Is an electrolyte that does not completely dissociate in solution. The solution will contain both ions and molecules of the electrolyte., 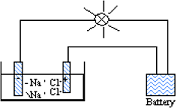 |
| Equation to Calculate Percent Water | 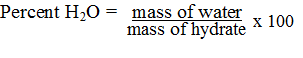 |