| A | B |
|---|
| Aldehyde | Any of a class of organic compounds containing the group ?CHO, which yields acids when oxidized and alcohols when reduced., 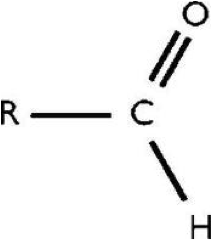 |
| Addition Reaction | The insertion of a small molecule directly into a double, triple covalent bond., 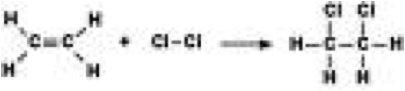 |
| Alcohol | Any of a series of hydroxyl compounds, the simplest of which are derived from saturated hydrocarbons, have the general formula and include ethanol and methanol., 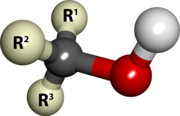 |
| Alkyl halide | A compound with the type formula RX, where R is an alkyl group and X is a halogen.,  |
| Aryl halide | In organic chemistry, a halogenoarene, haloarene, or aryl halide, is an organic compound in which a halogen atom is bonded to a carbon atom which is part of an aromatic ring.,  |
| Carbonyl Group | An organic compound containing the >CO group (see below). When a hydrogen atom is attached to the carbon, the resulting compound is known as an aldehyde. When only carbon atoms are attached, the resulting compound is known as a ketone. See also carboxyl group and amide group, 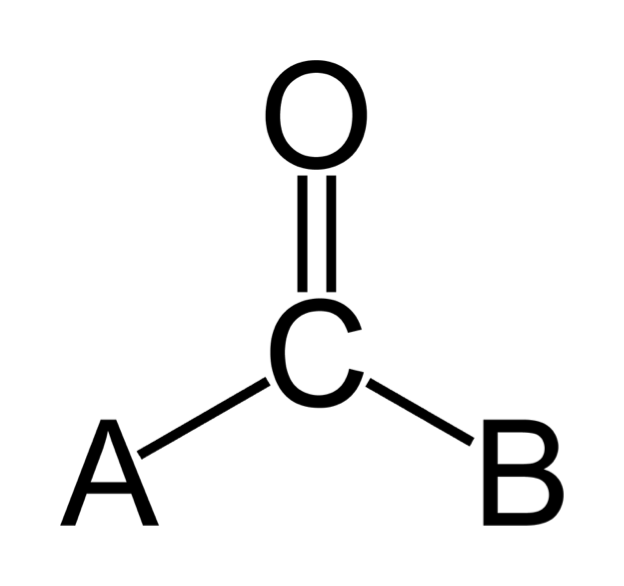 |
| Carboxyl group | The univalent radical -COOH; present in and characteristic of organic acids,  |
| Carboxylic acid | An organic acid characterized by one or more carboxyl groups, 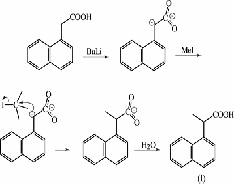 |
| Dehydrogenated reaction | A reaction in which two hydrogen atoms are removed from adjacent carbons of a saturated hydrocarbon, giving an unsaturated hydrocarbon, 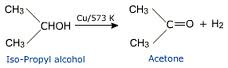 |
| Denatured alcohol | Ethyl alcohol that is unfit for drinking but is still useful for other purposes,  |
| Ester | Any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters derived from carboxylic acids are the most common., 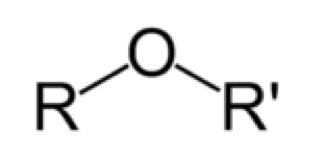 |
| Ether | Is a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R.,  |
| Fatty acid | A carboxylic acid with a long unbranched aliphatic tail (chain), which is either saturated or unsaturated,  |
| Fermentation | Process of deriving energy from the oxidation of organic compounds, such as carbohydrates, and using an endogenous electron acceptor, which is usually an organic compound, as opposed to Respiration where electrons are donated to an exogenous electron acceptor, such as oxygen,  |
| Functional group | Specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules,  |
| Halocarbon | One of various compounds of carbon and any of the halogens,  |
| Hydration reaction | Chemical reaction in which a hydroxyl group (OH-) and a hydrogen cation (an acidic proton) are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group., 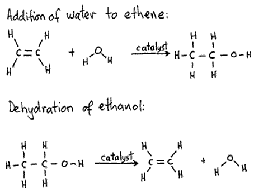 |
| Hydrogenation reaction | The addition of hydrogen to maleic acid to succinic acid,  |
| Hydroxyl group | This is a side group which is one hydrogen atom bonded to one oxygen atom,  |
| Ketone | Any of a class of organic compounds having a carbonyl group linked to a carbon atom in each of two hydrocarbon radicals, 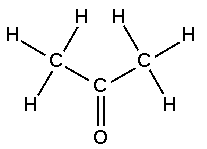 |
| Monomer | A simple compound whose molecules can join together to form polymers,  |
| Polymer | Naturally occurring or synthetic compound consisting of large molecules made up of a linked series of repeated simple monomers,  |
| Substitution reaction | The replacement of an atom or group of atoms in a compound by another atom or group.,  |