| A | B |
|---|
| Triple Covalent Bond | When two atoms are contributed to three pairs of electrons with each other., 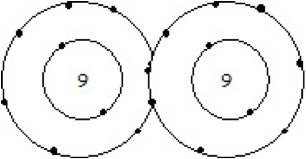 |
| Double Covalent Bond | The smallest particle of a substance that retains the chemical and physical properties of the substance and is composed of two or more atoms.,  |
| Resonance Structure | The property of a chemical compound having several possible structural arrangements of electrons in a molecule., 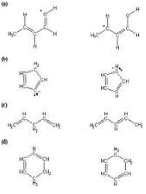 |
| Coordinate Covalent Bond | Strong type of intermolecular dipole-dipole attraction. Occurs between hydrogen and F, O or N., 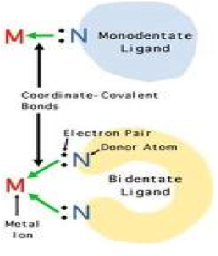 |
| Dipole – Dipole Interaction | Molecules composed only of two atoms, of either the same or different chemical elements.,  |
| Van Der Waals Forces | Is the attractive or repulsive force between molecules (or between parts of the same molecule) other than those due to covalent bonds or to the electrostatic,  |
| Bond Dissociation Energy | Is one measure of the bond strength in a chemical bond.,  |
| Bonding Orbitial | Molecular orbital located between two nuclei. Electrons in a bonding orbital stabilize a molecule., 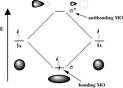 |
| Covalent Bond | Is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms, and other covalent bonds., 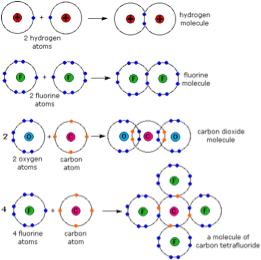 |
| Non Polar Covalent Bond | Intermolecular forces that exist between polar molecules. Active only when the molecules are close together. The strengths of intermolecular attractions increase when polarity increases.,  |
| Hybridization | Giant arrangements of matter in which atoms are covalently bonded together in a continuous two or three dimensional array., 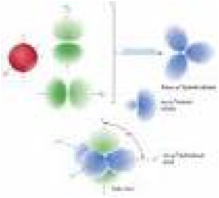 |
| Unshared Pair | Electrons that are not involved in bonding.,  |
| Network Solids | Covalent chemical bonds where two lobes of one involved electron orbital overlap two lobes of the other involved electron orbital,  |
| Sigma Bond | Bonds resulting from the head-on overlap of atomic orbitals, in which the region of electron sharing is along and (cylindrically) symmetrical to the imaginary line connecting the bonded atoms.,  |
| Molecular Orbital | An ion made up of two or more elements covalently-bonded.,  |
| Polyatomic Ion | An ion containing two or more elements chemically combined, 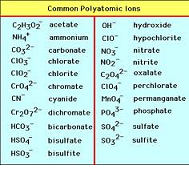 |