| A | B |
|---|
| Half-Reaction | Is either the oxidation or reduction reaction component of a redox reaction., 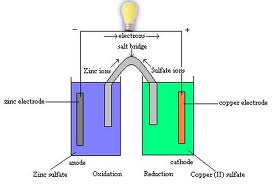 |
| Half-Reaction Method | To balance redox reactions, assign oxidation numbers to the reactants and products to determine how many moles of each species are needed to conserve mass and charge., 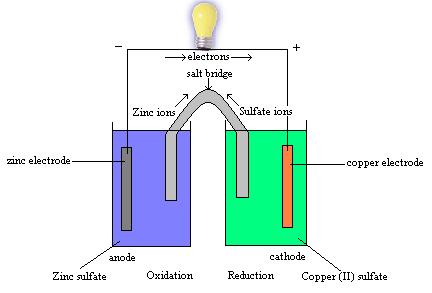 |
| Oxidation | The combination of a substance with oxygen.,  |
| Oxidation Number | The degree of oxidation of an atom or ion or molecule; for simple atoms or ions the oxidation number is equal to the ionic charge; "the oxidation number of hydrogen is +1 and of oxygen is -2", 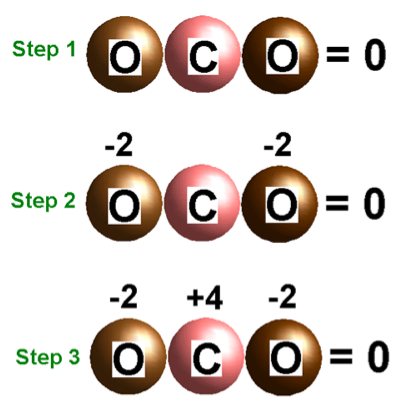 |
| Oxidation Number Change Method | Provides relatively an easy way to balance redox (including ionic redox) equations. The underline principle is that the gain in the oxidation number (number of electrons) in one reactant must equal to the loss in the oxidation number of the other reactant (Conservation of Electrons).,  |
| Oxidation Reduction Reaction | A reaction involving the transfer of electrons, 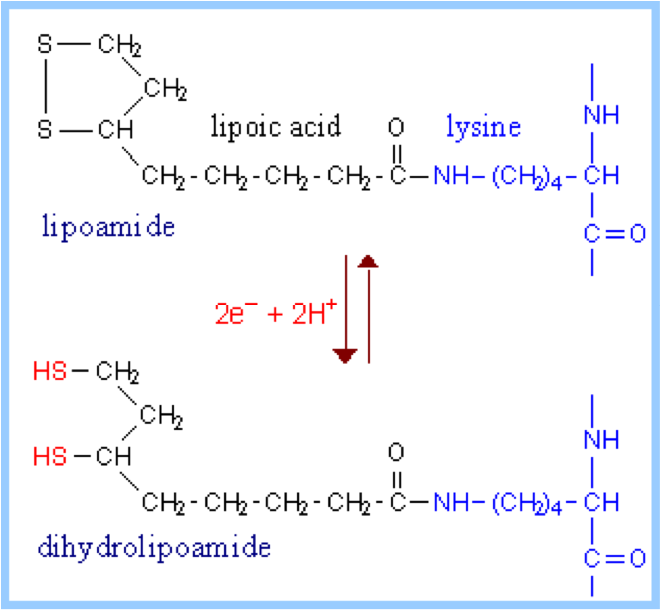 |
| Oxidizing agent | Can be defined as either: #a chemical compound that readily transfers oxygen atoms, or #a substance that gains electrons in a redox chemical reaction In both cases, the oxidizing agent becomes reduced in the process,  |
| Redox Reactions | Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is: C6H12O6 + 6 O2 ? 6 CO2 + 6 H2O,  |
| Reducing agent | A substance capable of bringing about the reduction of another substance as it itself is oxidized; used in photography to lessen the density of a negative or print by oxidizing some of the loose silver,  |
| Reduction | The amount by which something is lessened or diminished, 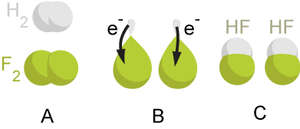 |