| A | B |
|---|
| Pi Bond | An intermolecular attraction force that exists between all molecules. These forces are the result of the movement of electrons which cause slight polar moments. Dispersion forces are generally very weak but as the molecular mass increases so does their strength.,  |
| Molecular Formula | An expression which states the number and type of atoms present in a molecule of a substance.,  |
| Hydrogen Bonding | An expression which states the number and type of atoms present in a molecule of a substance., 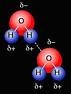 |
| Structural Formula | An expanded chemical formula that gives information about the arrangement of atoms and bonds in a molecule, 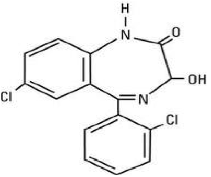 |
| Molecular Compound | An electrically neutral group of at least two atoms in a definite arrangement held together by very strong (covalent) chemical bonds., 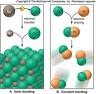 |
| Polar Bond | A type of covalent bond between two atoms in which electrons are shared unequally. Because of this, one end of the molecule has a slightly negative charge and the other a slightly positive charge., 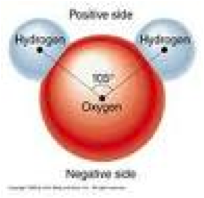 |
| Dipole | a special type of covalent bond in which the shared electrons come from one of the atoms only., 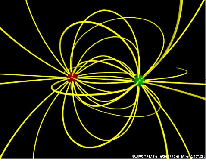 |
| Tetrahedral Angle | A solid angle bounded or enclosed by four plane angles., 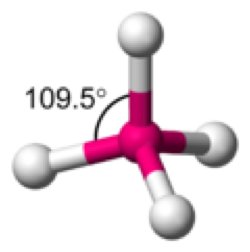 |
| Molecule | A separation of electric charge leading to a molecule having an electric dipole,  |
| Dispersion Force | A pair of equal and opposite electric charges or magnetic poles separated by a small distance, 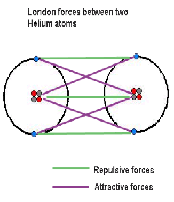 |
| VSEPR Theory | A model in chemistry used to predict the shape of individual molecules based upon the extent of electron-pair electrostatic repulsion, 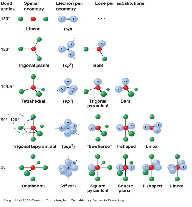 |
| Polar Molecule | A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair., 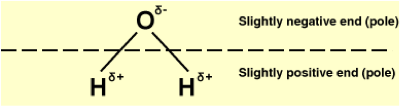 |
| Single Covalent Bond | A covalent bond between two atoms, formed by sharing a pair of electrons, 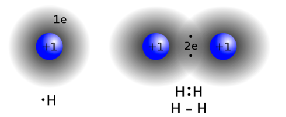 |
| Polar Covalent Bond | A bond in which electrons are shared between elements having a difference in electronegativity of between 0.5 and ~2.0., 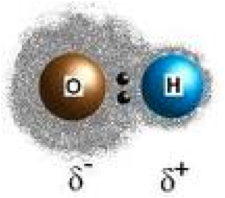 |
| Diatomic Molecule | A bond between two atoms in which both atoms share one electron, this electron forming a link between them., 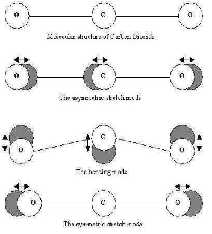 |
| Hydrogen Bonds | Is the attractive interaction of a hydrogen atom with an electronegative atom, like nitrogen, oxygen or fluorine,  |