 |
Java Games: Flashcards, matching, concentration, and word search. |
 |
 |
| A | B |
|---|
| Periodic Table | A tabular arrangement of the elements according to their atomic numbers so that elements with similar properties., 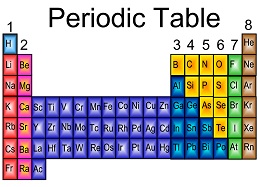 |
| Group | A vertical column in the periodic table; also called a family, 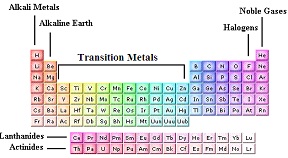 |
| Family | A group of elements with similar chemical properties,  |
| Period | The elements in a horizontal row of the periodic table., 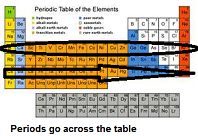 |
| Non-Metal | Any of a number of elements, such as oxygen or sulfur, that lack the physical and chemical properties of metals.,  |
| Metal | An alloy of two or more metallic elements.,  |
| Noble Gas | Any of the elements in Group 8 of the periodic table, including helium, neon, argon, krypton, xenon, and radon, which are monatomic and with limited exceptions chemically inert.,  |
| Halogen | Any of a group of five chemically related nonmetallic elements including fluorine, chlorine, bromine, iodine, and astatine.,  |
| Alkali Metal | Any of a group of soft, white, low-density, low-melting, highly reactive metallic elements, including lithium, sodium, potassium, rubidium, cesium, and francium.,  |
| Transition Metal | Any of the metallic elements within Groups 3 to 12 in the Periodic Table that have an incomplete inner electron shell and that serve as transitional links between the most and the least electropositive in a series of elements. They are characterized by multiple valences, colored compounds, and the ability to form stable complex ions.,  |
| Lanthanide | The set of chemically related elements with properties similar to those of lanthanum, with atomic numbers from 57 to 71; the rare-earth elements.,  |
| Actinide | The actinide or actinoid series encompasses the 14 chemical elements with atomic numbers from 90 to 103, thorium to lawrencium.,  |
| Valence Electron | Are the outermost electrons of an atom, which are important in determining how the atom reacts chemically with other atoms., 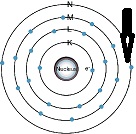 |
| Ionic Compound | Is a chemical compound in which ions are held together in a lattice structure by ionic bonds., 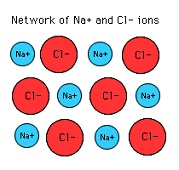 |
| Covalent Compound | A molecule formed by covalent bonds, in which the atoms share one or more pairs of valence electrons., 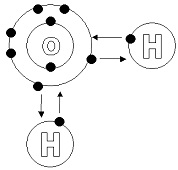 |
| Electronegativity | Is a chemical property that describes the ability of an atom (or, more rarely, a functional group) to attract electrons (or electron density) towards itself., 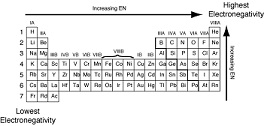 |
|
 |
 |
|
|
|
| |