| A | B |
|---|
Who developed this model?, 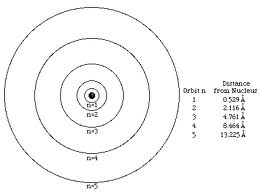 | Bohr |
Who developed this model?,  | Dalton |
Who developed this model?, 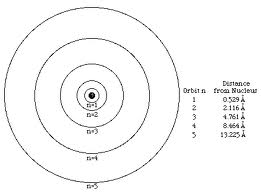 | Bohr |
Who developed this model?, 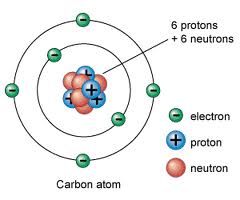 | Bohr |
Who developed this model?, 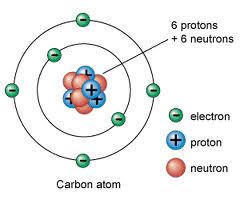 | Bohr |
Who developed this model?,  | Dalton |
Who developed this model?, 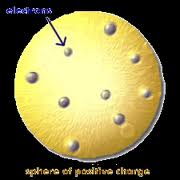 | Thomson |
Who developed this model?, 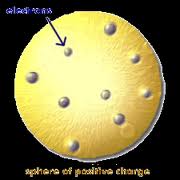 | Thomson |
Who developed this model?, 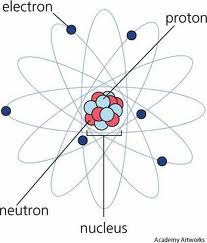 | Schrodinger |
Who developed this model?, 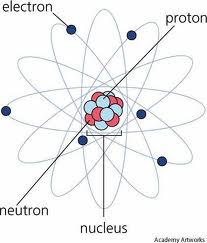 | Schrodinger |
Who developed this model?, 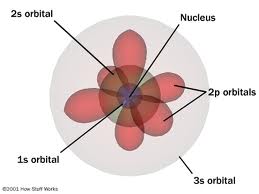 | Schrodinger |
Who developed this model?, 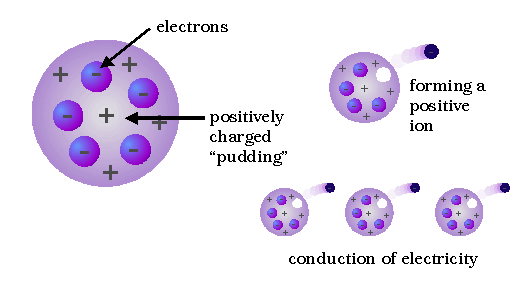 | Thomson |
Who developed this model?, 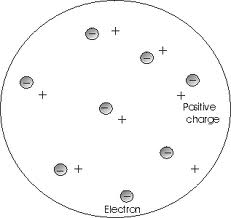 | Thomson |
| Discovered nucleus full of positively charged particles, and negative charges surrounded it | Ernest Rutherford |
| Electrons move in large empty space of an atom in set orbits | Niels Bohr |
| Discovered nucleus and coined term proton | Rutherford |
| 1911 Scientist contributing to Atomic Theory | Rutherford |
| 1913 Scientist contributing to Atomic Theory | Niels Bohr |
| Planetary Model – electrons orbit at set distance determined by energy level | Niels Bohr |
| Most of an atom is empty space except for nucleus | Rutherford |
| Electrons form a cloud not set orbitals | Schrodinger |
| All elements are composed of atoms. Atoms are indivisible and indestructible particles. | John Dalton |
| First person to realize that electrons exist on ‘energy levels’ and you can predict where the electron can be found | Niels Bohr |
| Determined that most of the mass of an atom is found in the nucleus | Rutherford or Chadwick |
| 1932 Scientist contributing to Atomic Theory | Chadwick |
| Discovered neutrons in nucleus | Chadwick |
| Hypothesized neutrons in nucleus | Rutherford |
| 1930s til present day - Scientist contributing to Atomic Theory | Schrodinger |
| Protons and neutrons are each made up of three quarks (made of strings) | Schrodinger |
| Electrons are found in orbits based on energy level | Niels Bohr |
| 1803 - Scientist contributing to Atomic Theory | John Dalton |
| Said that an atom is solid material with electrons scattered throughout | Thomson |
| Said that an atom is solid material with electrons scattered throughout | John Dalton |
| Plum Pudding Model “electrons scattered like raisins in plum pudding” | JJ Thomson |
| Discovered electrons but called them corpuscles | JJ Thomson |
| Compounds are formed by the joining of atoms of two or more elements | John Dalton |
| Atoms of same element all look alike and atoms of different elements look different | John Dalton |
| First person to realize that matter is made of atom | Democritus |
| Greek word “indivisible” | Democritus |
| Recieved a Nobel Prize for electricity in gases | JJ Thomson |
| School teacher | John Dalton |
| Discovered atoms are made of charged particles | JJ Thomson |
| Discovered electrons | JJ Thomson |
| Discovered isotopes | JJ Thomson |
| Discovered the atom is mostly empty space | Rutherford |
| Planetary Model | Bohr |