| A | B |
|---|
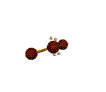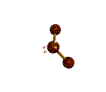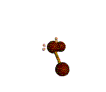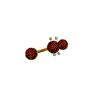 | BENT about 109.5 degrees |
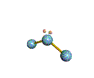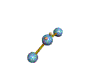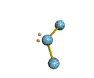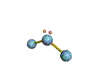 | BENT about 120 degrees |
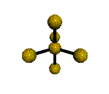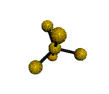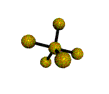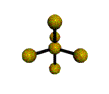 | TRIGONAL BIPYRAMIDAL 120, 180, 90 degrees |
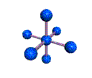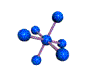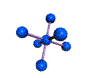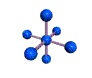 | OCTAHEDRAL 90, 180 degrees |
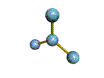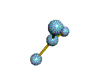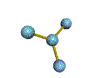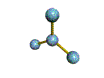 | TRIGONAL PLANAR 120 degrees |
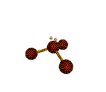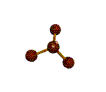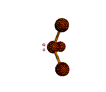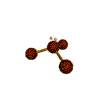 | TRIGONAL PYRAMIDAL about 109.5 degrees |
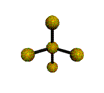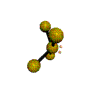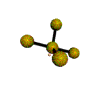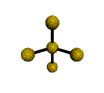 | SEESAW about 120, 180, 90 degrees |
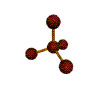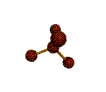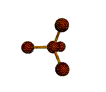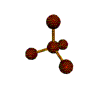 | TETRAHEDRAL 109.5 degrees |
| Any 2 atom molecule, any 3 atom molecule in a straight line | LINEAR no actual bond angle if only 2 atoms; 180 degrees if 3 atoms |
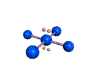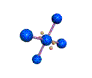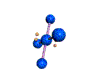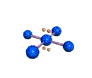 | SQUARE PLANAR 90, 180 degrees |