| A | B |
|---|
| The first people to hypothesize about the nature of matter were the _______. | ancient Greeks |
| The first person to name the basic particle an "atom" was the ancient Greek philosopher ______. | Democritus |
| The Greek philosopher ______ did not believe in atoms. Instead, he believed all matter was continuous, an opinion that was accepted for the next 2000 years. | Aristotle |
| Which law states that "mass is neither created nor destroyed during ordinary chemical reactions or physical changes." | The law of conservation of mass |
| Mass is neither _____ nor ______ during ordinary chemical reactions or physical changes. | created, destroyed |
| Thomson's ________ experiments measured the charge-to-mass ratio of an electron. | cathode-ray tube,  |
________ was able to determine the charge-to-mass ratio of an electron with his cathode-ray tube experiments.,  | J.J. Thomson,  |
| Ernest Rutherford's _________ experiments led to the discovery of the nucleus. | gold foil,  |
_______ discovered the nucleus with his gold foil experiment (1911).,  | Rutherford,  |
Most of the alpha particles that Rutherford shot at the thin gold foil ______, indicating that atoms are mostly empty space.,  | passed through,  |
Most of the alpha particles that Rutherford shot at the thin gold foil passed through, indicating that atoms are mostly _______.,  | empty space,  |
The fact that a small percentage of the alpha particles that Rutherford shot at the thin gold foil seemed to bounce back was evidence for the presence of ______ in atoms.,  | a small dense nucleus,  |
The fact that a small percentage of the alpha particles that Rutherford shot at the thin gold foil seemed to ________ was evidence for the presence of a small dense nucleus in atoms.,  | bounce back,  |
| Elements with 3 electron shells would be found in the ____ period. | 3rd |
Elements with the same number of electron shells as in the picture below can be found in the ___ period., 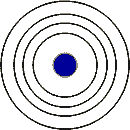 | fourth,  |
| Who created the first periodic table in 1869? | Mendeleev |
| Repeating properties are also called ____ properties. | periodic |
| The first periodic table was based on the ordering of elements by increasing _______. | atomic mass (today, it goes by increasing atomic number) |
| The first periodic table had several empty spaces because ______. | the elements that fit in those empty spaces hadn't been discovered yet (they were within the next 15 years) |
| Who was the first person to organize the periodic table according to increasing atomic number? | Moseley (in 1911); Mendeleev had organized his periodic table by increasing atomic mass |
| Thomson proposed the ________ model of the atom in which he envisioned positive and negative charged particles of the atom to be spread out evenly. | plum pudding, 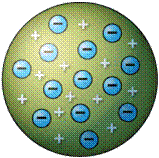 |
| The ________ model of the atom postulated that electrons travel in fixed orbits around the nucleus. | Bohr |
| Electrons must _______ energy to move to orbits that are further away from the nucleus | absorb |
| Electrons _______ energy when they move to lower energy levels. | emit (same as "lose" or "give off" energy) |