| A | B |
|---|
| Democritus (1) | Matter is made up of small hard particles that cannot be divided. |
| Democritus (2) | He named the small particle “Atomos”, or Atom. |
| Democritus (3) | Atoms are infinite, always moving, and can join together. |
| Democritus (4) |  |
| John Dalton (1) | All matter is made of atoms. |
| John Dalton (2) | Atoms of an element are identical. |
| John Dalton (3) | Atoms cannot be divided, created, or destroyed. |
| John Dalton (4) | Atoms can combine to form compounds. |
| John Dalton (5) | Chemical reactions combine, rearrange, or separate atoms. |
| John Dalton (6) “Billiard Ball Model” |  |
| JJ Thomson (1) | He discovered the electron. |
| JJ Thomson (2) | He discovered that atoms are made of smaller particles. This means the atom can be divided. |
| JJ Thomson (3) | He discovered the atom also had a positive charge. |
| JJ Thomson (4) | He thought the atom was made from a positive substance with negative particles spread inside. |
| JJ Thomson (5) “Plum Pudding Model” | 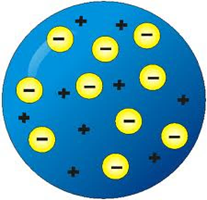 |
| Rutherford (1) | Most of the mass of the atom is concentrated at the center of the atom. |
| Rutherford (2) | He discovered the nucleus. |
| Rutherford (3) | All of the atom’s positive particles are in the nucleus. |
| Rutherford (4) | The nucleus is in the center of the atom and electrons are scattered around the open space. |
| Rutherford (5) “Nuclear Model” | 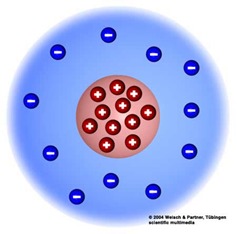 |
| Niels Bohr (1) | The energy of each electron is related to the electron’s path around the nucleus. |
| Niels Bohr (2) | He discovered electrons were located on energy levels or orbits. |
| Niels Bohr (3) | The number of energy levels that are filled in an atom depends on the number of electrons. |
| Niels Bohr (4) | 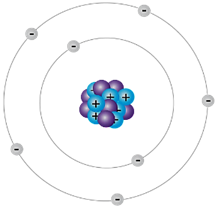 |
| James Chadwick (1) | He discovered that the reason for mass differences was that the nuclei of atoms contain a particle with no electric charge. |
| James Chadwick (2) | He discovered the neutron. |
| James Chadwick (3) | He calculated the mass of a neutron. |
| James Chadwick (4) | His discovery resulted in a model of the atom that is easy to understand. |
| James Chadwick (5) | 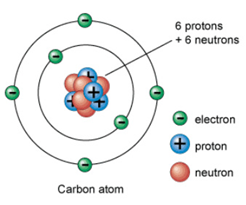 |
| Schrödinger (1) | He used math equations to describe the chances of finding an electron in one specific location. |
| Schrödinger (2) | Electrons do not move around the atom in an orbit. |
| Schrödinger (3) | An atom has a small positively charged nucleus surrounded by a cloud of electrons. |
| Schrödinger (4) | The probable location of an electron is based on how much energy the electron has. |
| Electron Cloud Model | 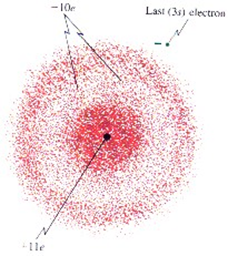 |
| Quantum Mechanical Model | 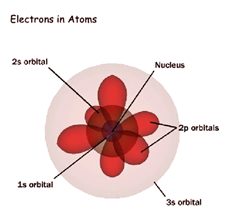 |