| A | B |
|---|
| aromatic acide is called | benzoic acid |
| physical property of 1-9 saturated carbon atoms | liquid |
| physical property more than 9 saturated carbon atoms | odorless solid |
| physical property of unsaturated carboxylic acids | liquids |
| Carboxylic solubility 1-5 carbon atoms in HOH (water H20) | soluble (the H-bonds with HOH) |
| carboxylic acids are | weak acids |
| Esterification | HOH is produced from the (-OH) removed from the carboxylic acid and an H-lost by the alcohol |
| Esters | produced in reaction bewteen carboxylic acid and alcohol |
| Ester functional group | 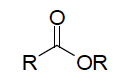 |
| When naming an ester the first word is the name of the ________ and the 2nd word is the name of the _________ with the ending ( -oic acid ) replaced with ( -ate ) | alkyl of the alcohol; carboxylic acid |
| Physical property of esters with 1 to 9 carbons are | liquid |
| Physical property of esters with lower molecular weights are | soluble in HOH (water) |
| An ester is neither an _____ or a ______ | acid; Base |
| Acid hydrolysis produces | carboxylic acid and an alcohol |
| saponification | a reaction of a long-chain fatty acid and NaOH (makes soap) |
| ethyl alcohol in wine exposed to oxygen in air produces | vinegar |
| carboxylic acids can be prepared from | primary alcohols or aldehydes |
| the reason for the higher boiling points of carboxylic acids is that 2 carboxylic acids form hydrogen bonds between their carboxyl group results in a _______ | Dimer |
| When a dimer is formed the mass of the carboxylic acid is | doubled |
| Neutralization of carboxylic acid produces | carboxylate salt and water |
| During exercise | pyruvic acid is converted to lactic acid |
| Citric acid | gives the sour taste to citrus fruits |
| Carboxylic acid reacts with an alcohol to form an | Ester |
| The aroma and flavor of many ______ are due to esters | fruits |
| Esters with 2 to 5 carbons are __________ in HOH (water) | soluble |
| solubility of esters in water ______ as the number of carbon atoms_________ | decrease; increase |