| A | B |
|---|
How many electrons does a Fluorine atom have?,  | 9 |
How many electrons does a Helium atom have?, 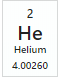 | 2 |
Looking at the carbon atom - does it have a positive, negative, or neutral charge?,  | neutral |
How many electrons does the Carbon atom have?,  | 6 |
What is the mass of the Carbon atom?,  | 12 |
How many valence electrons does Phosphorus have?,  | 5 |
How many valence electrons does Helium have?,  | 2 |
How many valence electrons does Neon have?,  | 8 |
Name the element with 16 Protons.,  | Sulfur |
Name the atom with 17 Electrons,  | Chlorine |
What is the Atomic Mass for Argon?,  | 39.948 |
What do Oxygen and Sulfur's outer electrons have in common?,  | 6 valence electrons |
| The rows (left to right) in the periodic table are called... | Periods |
| The columns (up and down) in the periodic table are called... | Groups |
| Atoms are placed in the same Group when they have this in common: | Number of valence electrons |
| Atoms are placed in the same Period when they have this in common: | Number of electron levels |