| A | B |
|---|
| The changing of water from gas form (water vapor) to liquid form is called ____. | condensation |
| The changing of water from liquid form to gas form (water vapor) at temperatures below boiling is called ___. | evaporation |
| Solid water is also called _____ and _____, | ice and snow |
| Water in the gas phase is called _____. | Water vapor |
| The process called _____ changes water from the solid to the liquid form. | melting |
| The process called _____ changes water from the liquid to the solid form. | freezing |
| The process called _____ changes water from the solid straight to the gaseous form. | sublimation |
| The process called _____ changes water from the gaseous form straight to the solid form. | deposition (the frost on your car early in the morning is an example) |
| Water molecules in the solid and liquid phases are held close together by __________ bonds. | hydrogen bonds (Notice how the big red hydrogen atom of a water molecule is partially negatively charged while the smaller hydrogen atoms in a water molecule are partially positively charged. Opposites attract which is why one water molecule forms hydrogen bonds with another water molecule as long as the positive and negative parts of the two molecules are close to each other), 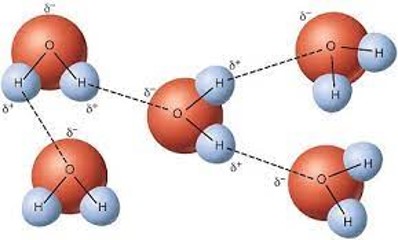 |
| Water molecules in the _____ and liquid phases are held closely together by hydrogen bonds. | solid and liquid (The molecules in the gas phase are too far apart from each other and moving too fast to establish hydrogen bonds between them), 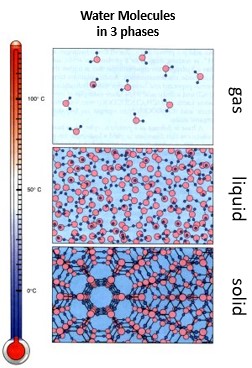 |
| Water in the ______ phase has the most hydrogen bonds between water molecules. | solid (Notice how the water molecules in the solid phase are lined up in a pattern that allows them to maximize the hydrogen bonds that occur between the partially negatively charged oxygen atom of one water molecule and the partially positively charged hydrogen atoms of a different water molecule),  |
| When water changes phases to a state that requires hydrogen bonds to break, energy gets _______. | absorbed (because it require energy to break bonds) |
| When water changes phases to a state that requires more hydrogen bonds to form, energy gets _______. | released (anytime a bond forms, energy gets released. It's the opposite of what happens when bonds break. Energy needs to be absorbed in order to break bonds) |
| Changing phases from _____ to _____ or ______ to _______ causes energy to be absorbed. | solid to liquid, liquid to gas (this is because hydrogen bonds need to be broken in order for these phase changes to occur, and breaking bonds requires energy) |
| Changing phases from _____ to _____ or ______ to _______ causes energy to be released. | gas to liquid, liquid to solid (That's why you get burns if you let water vapor from a steaming pot of boiling water condense on your skin. It's also why when water in clouds freeze to form snow, the temperature tends to warm up a little) |
| The heat energy that is either absorbed or released by a substance during a phase change is called ______. | latent heat (notice how much latent heat needs to be absorbed by liquid water to turn it into water vapor. When water vapor condenses, it releases just as much heat as it needed to absorb when it was changing from liquid to vapor), 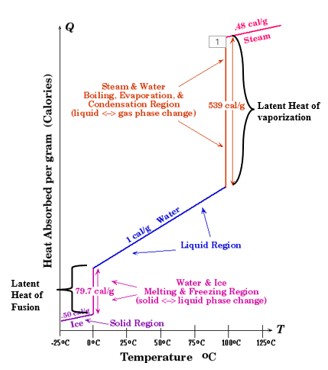 |
| Where does the heat energy that is needed to evaporate sweat off your body come from? | Your body (That's why sweating cools your body as long as the air is dry enough for evaporation to occur) |
| Water vapor in the atmosphere is known as _________. | humidity |
| Air can only hold a certain amount of water vapor. When air gets to the point that it can’t hold anymore water vapor, the air is said to be __________. | saturated |
| ________ air can hold more water vapor than ________ air. | Warm, cold |
| ________ air can't hold as much water vapor as ________ air. | Cold, warmer |
| When air is saturated, any additional water vapor added to the air undergoes ________. | Condensation |
| Air that is not saturated can become saturated if it’s temperature _____ enough | drops enough (This is why fog can form if warm moist air comes in contact with a cold air mass or a cold surface like snow) |
| The temperature at which air with a certain amount of humidity needs to drop to in order for it to become saturated is called its _______. | dew point |
| When air near the ground drops in temperature to its dew point, ______ will appear in the air and ___ will appear on the ground.. | fog, dew (Actually, dew can start forming a little bit before the air reaches the dew point temperature because the ground can cool a little faster than the air due to radiational cooling) |
| When air temperatures drop to reach the air's dew point, what is its relative humidity? | 100% |
| _______ humidity is a measure of the actual mass of water vapor in a cubic meter of air. | Absolute |
| _______ humidity is the amount of water vapor in air at a given temperature compared to what that air could hold if it was to become saturated. It is expressed as a percentage. | Relative |
| The closer air is to having a relative humidity of 100%, the harder is for water to ____________. | evaporate (That is why you don’t get the same cooling effect from sweating when the relative humidity is high) |
| TRUE or FALSE: If you do nothing other than cool down an air mass, it will feel more humid. | TRUE (Since colder air can't hold as much water vapor as warmer air, the relative humidity of cooling air gets higher as air cools. The relative humidity levels usually go up at night even though it usually gets cooler) |
Look at the chart. If it was 100 degrees on a really dry day out in the desert (relative humidity at 10%), what would the temperature feel like to you?, 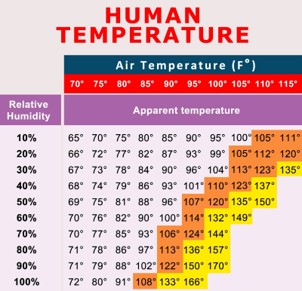 | 95 degrees (Find 100 degrees in the red area. Then find 10% relative humidity in the column on the left. Where those two intersect is where you read the "apparent humidity" which is what the temperature feels like. When it is really dry out, your sweat evaporates so fast that it feels a little cooler than the actual temperature).,  |
Look at the chart. If it was 100 degrees on a hot humid summer day in Philadelphia (relative humidity of 60%), what would the temperature feel like to us?,  | 132 degrees (Yah, it would feel like a sauna. Just find the column in the red area with 100 degrees, then drop down to the row that matches up with 60% relative humidity),  |
Look at the chart. When it feels like it's 84 degrees, the actual temperature is _____ if the relative humidity is _____.,  | 85, 30% (Just find the only place it says 84 degrees on the chart. Then look to the left to find the relative humidity, and look above to find the actual temperature),  |