| A | B |
|---|
| Name the first 10 alkanes. | methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, decane |
| Straight chain hydrocarbons with double bonds are called ______. How would you name one with 3 carbons? | alkenes, propene |
| Straight chain hydrocarbons with triple bonds are called _____. How would you name one with 2 carbons? | alkynes, ethyne |
| How can you make an ester? | Reacting a carboxylic acid and an alcohol in the presence of heat and an acid catalyst will produce a dehydration synthesis that makes an ester. |
What is the name of this molecule?, 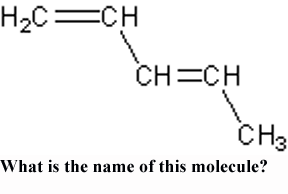 | 1,3-Pentadiene,  |
What is the name of this molecule?, 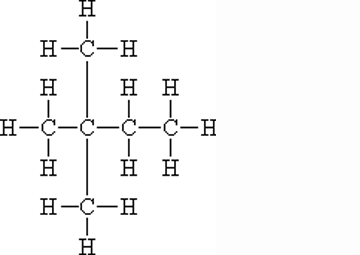 | 2,2-dimethyl-butane,  |
What is the name of this molecule?,  | 2,3-dimethyl-butane,  |
What is the name of this ion and what is it's charge?, 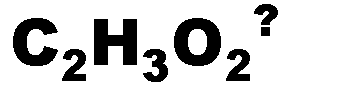 | Acetate (-1), 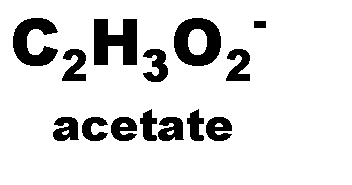 |
What kind of functional group is this?, 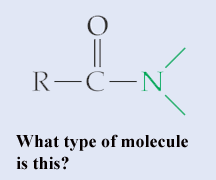 | It's an amide (notice that it includes the double bonded oxygen), 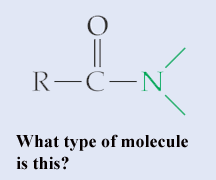 |
What is the name of this ion and what is it's charge?, 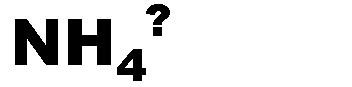 | Ammonium (+1),  |
What type of molecule is this?, 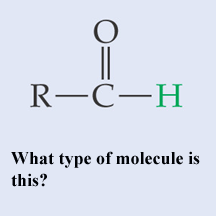 | A basic aldehyde,  |
What is the name of this ion and what is it's charge?, 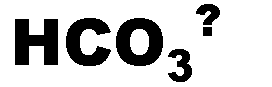 | bicarbonate (-1),  |
What is the name of this ion and what is it's charge?, 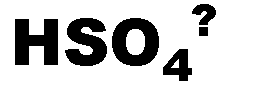 | bisulfate (-1),  |
What is the name of this ion and what is it's charge?, 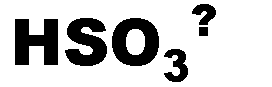 | bisulfite (-1),  |
What is the name of this ion and what is it's charge?, 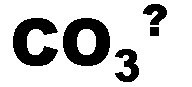 | Carbonate (-2),  |
What type of molecule is this?,  | carboxylic-acid,  |
What is the name of this ion and what is it's charge?, 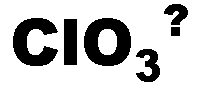 | chlorate (-1),  |
What is the name of this ion and what is it's charge?,  | Chromate (-2),  |
What is the name of this ion and what is it's charge?,  | cyanide (-1),  |
What is the name of this ion and what is it's charge?, 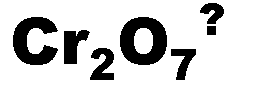 | dichromate (-2), 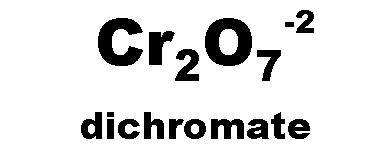 |
What type of molecule is this?, 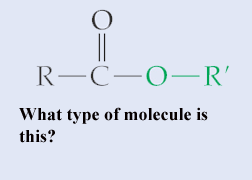 | An ester, 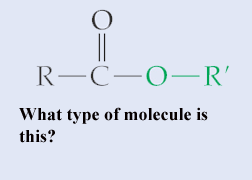 |
What type of molecule is this and what is it's name?, .gif) | Ester (propyl-ethanoate)., .gif) |
What type of molecule is this and what is it's name?, .gif) | Ether (dimethyl-ether)., .gif) |
What type of molecule is this and what is it's name?, -copy.gif) | aldehyde (hexanal), -copy.gif) |
What is the name of this ion and what is it's charge?,  | hypochlorite (-1),  |
What type of molecule is this and what is it's name?, .gif) | ketone (2-butanone), .gif) |
What type of molecule is this?, 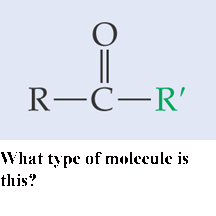 | Ketone, 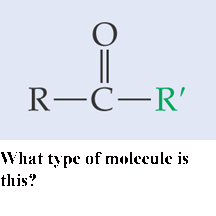 |
What is the name of this ion and what is it's charge?, 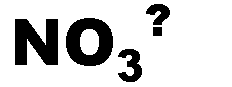 | Nitrate (-1),  |
What is the name of this ion and what is it's charge?, 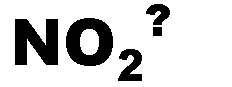 | nitrite (-1),  |
What is the name of this ion and what is it's charge?, 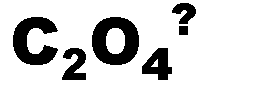 | oxalate (-2), 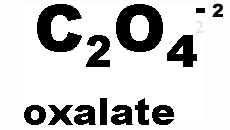 |
What is the name of this ion and what is it's charge?, 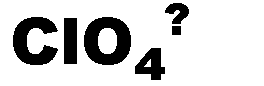 | perchlorate (-1),  |
What is the name of this ion and what is it's charge?,  | permanganate (-1),  |
What is the name of this ion and what is it's charge?, 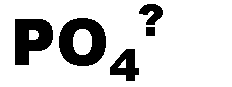 | phosphate (-3),  |
What is the name of this ion and what is it's charge?, 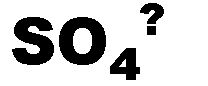 | sulfate (-2),  |
What is the name of this ion and what is it's charge?, 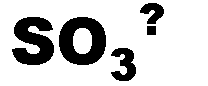 | sulfite (-2),  |
| How would you find out the temperature change of a substance if you added a certain amount of heat to a certain amount of the substance? | Delta T = q / mc (where q=heat, m=mass and c=specific heat). So change in temperature is = heat (calories) divided by (mass x specific heat) |
| What are the units of measurement for specific heat? | cal/(g*C) |
| How can you figure out the empirical formula of a substance if given the percent composition? | Make the percentages = grams. Then figure out the number of moles of each element. Then multiply them until you get all whole numbers. |
| What is an empirical formula? | Like a molecular formula reduced to the lowest common denominator (ex: glucose would be CH2O. |
| The term that describes disorder or randomness is ___. | entropy |
| Three words that can be associated with high entropy are ____, _____, and ___. | low energy, stability, disorder |
| Which symbol is associated with entropy? | S |
| What does a positive delta S mean? | Reaction gained entropy, products became more disordered |
| What does a negative delta S mean? | Reaction lost entropy, products became more ordered (less disordered) |
| Enthalpy can be associated with ____. | chemical potential energy |
| Which symbol is associated with enthalpy | H |
| A negative delta H is caused by a(n) ____ reaction. | Exothermic reaction (remember, delta H means change in enthalpy) |
| A positive delta H is caused by a ____ reaction. | endothermic reactions (remember, delta H means change in enthalpy) |
| How do you determine delta H for a reaction if given the delta H of formation (heat of formation) of the products and reactants. | Delta H of rxn = delta H of formation (products) - delta H of formation (reactants). Remember that the delta H of formation for an element (including diatomic elements) is zero. |
| Delta G is associated with ___. | Gibbs free energy |
| Delta G = ? | Delta G = delta H - T*delta S (Gibbs free energy is equal to Enthalpy minus Temperature (in degrees Kelvin) times change in entropy. |
| A change in Gibbs free energy in the negative direction means ___. | A change in Gibbs free energy in the negative direction means the reaction will be spontaneous in the forward direction. |
| The Heisenberg Principle basically means that ___. | One cannot know an electron's position and velocity at the same time. |
| "One cannot know an electron's position and velocity at the same time" is stated by the ___. | Heisenberg uncertainty principle |
| De Broglie's Hypothesis states that ___. | Everything can be thought of as a wave or a particle. |
| "Everything can be thought of as a wave or a particle" is stated in ___. | De Broglie's hypothesis |
| An element in the s area of row n puts the outer shell electrons into the ____ s subshell. | n |
| An element in the p area of row n puts the outer shell electrons into the ____ p subshell. | n |
| An element in the d area of row n puts the outer shell electrons into the ____ d subshell. | n-1 |
| An element in the f area of row n puts the outer shell electrons into the ____ f subshell. | n-2 |
| Outer shell electrons of Helium are in the ____ subshell. | 1s |
| If an element has an atomic number greater than ____, it has electrons in the f subshell | 57 |
| The electrons in an atoms outermost shell are called ___. | valence electrons |
| Alpha decay involves the ejection of a ____. | helium nucleus |
| When alpha decay occurs, what happens to the atomic number and mass number? | Atomic number is reduced by 2 and mass number is reduced by 4 |
| A beta particle is identical to a(n) ___. | electron |
| What happens to atomic and mass numbers with beta decay? | Atomic number goes up by one but mass number remains the same (a neutron is converted into a proton when it ejects a beta particle). |
| What happens to atomic and mass number when a nucleus emits a positron? | Atomic number goes down by one but mass number remains the same (a proton loses it's positive charge to become a neutron) |
| Group 1 elements are known as the ____. | alkali metals |
| Group 2 elements are known as the ___. | alkali earth metals |
| Group 7 elements are known as the ____. | halogens |
| Group 8 elements are known as the ___. | noble gases |
| What is the periodic trend for ionization energy? | Ionization energy (energy needed to take one electron away, thus ionizing it) goes up as you go across and down as you go down). Increases as you head northeast. |
| What is electronegativity? | The amount of "pull" that an atom has on the electrons of another atom. |
| What is the periodic trend for electronegativity? | Goes down as you go down and up as you go across. (Increases as you head northeast) |
| What is the periodic trend for atomic radius? | Radius goes down as you go across and up as you go down (increases as you head southwest) |
| What is metallic character? | Metallic character is the measure of how much an atom likes to give up electrons to form a positive ion. |
| What is the periodic trend for metallic character? | Metallic character increases as you go down and decreases as you go across. Increases in a southwest direction. |
| What is a metallic bond? | A metallic bond is a bond between metals in which the metals allow their valence electrons to roam about freely, like a sea of mobile electrons. |
| What is bond energy? | The amount of energy needed to break a bond. |
| Describe how you can predict the delta H of a reaction using bond energies. | Delta H for the reaction will equal the bond energies of the reactants minus the bond energies of the products. Thus, if it requires more energy to break the bonds of the products than the reactants, you will get a negative delta H, meaning that the reaction is exothermic. |
| What are the bond angles of a perfect tetrahedron, like CCl4 | 109.5 degrees |
| What is the bond angle in a water molecule? | What molecules are bent at about 105 degrees. |
| Which two elements violate the octet rule, and how many electrons are they happy with? | Beryllium (Be) is happy with 4 valence electrons. When it serves as the central atom in a compound, the molecule is linear. Boron strives to have 6 valence electrons. When boron acts as the central atom in a molecule, that shape is generally trigonal planar |
| How do you convert "torr" into mmHg? | 1 torr = 1 mmHg |
| What does 1 atmosphere equal? | 1 atmosphere = 760 mmHg = 760 torr. |
| What are the two characteristics/assumptions about an ideal gas? | 1) Its molecules are not attracted to each other. 2) It's molecules occupy an insignificant volume compared to the volume of the container holding the gas. |
| What is the ideal gas equation? | PV = nRT (where n=number of moles of the gas, and R = the ideal gas constant = .082 L-atm/mol-K) |
| What are partial pressures and how do you figure them out? | Partial pressures come into play when you have more than one gas in a container. The total pressure in the container is = to the sum of the parital pressures of the gases in the container. The partial pressures of each gas are directly proportional to the number of moles of that gas compared to the total number of moles of gases in the container. |
| What is an intermolecular force? | a weak bond of attraction between molecules (ie. - hydrogen bonds between water molecules) |
| What is an intramolecular force? | Bonds between atoms in a molecule (ie - covalent bond) |
| What is a network solid? | A covalently bonded substance that does not consist of individual molecules (ie. - SiO2 which can be one big chunk of Si and oxygen bonded together with covalent bonds) |
| What is a Van der Waals force and what are it's three forms? | Van der Waals forces are week intermolecular forces. Three types are dipole-dipole (like cohesion in water), dipole induced-dipole, and induced dipole-induced dipole) |
| What is "heat of fusion"? | The amount of heat required to move a substance from its solid to its liquid phase. |
| What is "heat of vaporization"? | The amount of heat required to move a substance from its liquid to its gaseous phase. |
| How does an increase in pressure affect a substance's melting and boiling point? | Increases both the melting and boiling point |
| How does a decrease in pressure affect a substance's melting and boiling point? | Decreases both the melting and boiling point |
| What is the relationship between vapor pressure and evaporation? | The higher the vapor pressure of a liquid, the more easily it evaporates at temperatures below the boiling point |
| What happens to the entropy of a substance when it undergoes a phase change? | Phase changes that involve going from a solid to a liquid or a liquid to a gas have a positive entropy (increase in disorder) |
| What is molarity? What is its symbol? | moles of solute per liter of solution. The symbol for molarity is M |
| What is molality? What is its symbol? | number of moles of solute per kilogram of solvent. The symbol for molality is m. |
| How do you figure boiling point elevation and freezing point depression of a solution due to increasing its solute concentration? | Delta T = km where k is a constant that depends on the solvent (not the solute) and m is the molality of the dissolved particles in the solution. For example, in a water solution, 1 mole of NaCl will increase the boiling point and decrease the freezing point twice as much as 1 mole of sucrose because NaCl produces two dissolved particles compared to sucrose. |
What is the equilibrium equation for the reaction below? When writing equilibrium equations, how do you decide which products or reactants to include?,  | Remember, only include those species whose concentrations can be varied. Only species that are gaseous or in solution belong in an equilibrium equation., 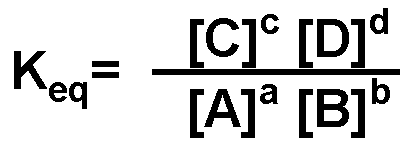 |
| If the equilibrium constant of a reaction is greater than 1, then | the forward reaction is favored |
| If the equilibrium constant of a reaction is less than 1, then | the reverse reaction is favored |
| Le Chatelier's principle basically says that | If, in a chemical equilibrium, some stress is imposed, the equilibrium will shift in a direction that relieves the stress (stress means crowding). This can apply to temperature and pressure as well. Just add temperature or pressure into the equation. For example, in the equation H + I + heat<---> J + K, if you add heat, you will drive the equilibrium formward. If we remove heat, then we drive the equilibrium back to the left. |
| What would happen if you added pressure to the following reaction when it is in equilibrium? N2 + 3H2 <--> 2NH3 | If you increase the pressure to the following equilibrium, the reaction will shift to the right to reduce the stress (Le Chatelier's principle) because 2 moles of ammonia gas take up less space than 1 mole of N2 gas + 3 moles of H2 gas. |
| What effect do catalysts have on equilibrium? | Catalysts have no effect on equilibrium. They can help equilibrium be achieved faster, but it won't affect the equilibrium. |
For the following slightly soluble solid in equilibrium with its aqueous products, what is the equilibrium equation?, 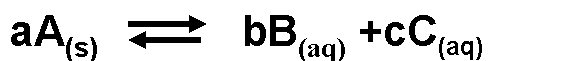 | ., 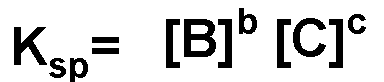 |
| pH = ____ | pH = - log of the H+ concentration |
| The equilibrium constant for the ionization of water = ____ | The equilibrium constant for the ionization of water = 1.0 X 10 to the -14 |
| If the hydroxide ion concentration is 10 to the -4, what is the hydrogen ion concentration? | 10 to the -10 (remember that the hydrogen ion concentration x the hydroxide ion concentration must equal 10 to the -14) |
| Non-metal oxides dissolve in water to form ____. | Non-metal oxides dissolve in water to form acids (examples include SO3 + H2O --> H2SO4 and CO2 + H2O --> H2CO3 |
| Metal oxides dissolve in pure water to form ___. | Metal oxides dissolve in pure water to form bases (for example, K2O + H2O --> 2KOH |
| Summarize the Arrhenius view of acids and bases. | Acids are substances that dissolve in water to form hydrogen ions and bases are substances that dissolve in water to form hydroxide ions. |
| Summarize the Bronsted-Lowry acid-base theory. | In the Bronsted-Lowry acid-base definition, the reactants always contain an acid and a base. The acid is the proton donor and the base is the proton acceptor. The products, since they can react in the reverse direction to reform those reactants by donating and accepting protons are also considered acids and bases, only they are referred to as conjugate acids and conjugate bases. For example, in the equation, NH3 + H2O <--> NH4+ plus OH-, the water is the acid and the NH3 is the base on the reactants side, while the ammonium ion is the conjugate acid and the hydroxide ion is the conjugate base on the products side. |
| In the Lewis acid-base definition, the acid is | In the Lewis acid-base definition, the acid is an electron pair acceptor (Remember, Lewis is a BOAR as in base is oxidized and acid is reduced) |
| In the Lewis acid-base definition, the base is | In the Lewis acid-base definition, the base is the electron pair donor (Remember, Lewis is a BOAR as in base is oxidized and acid is reduced) |
| What is the pH of a 0.1 Molar solution of a strong acid such as HCl? | Strong acids ionize completely, so the H+ concentration is 0.1 M = 1x10 to the -1 Molar = a pH of 1. (A 1.0 molar solution would have a pH of zero and a 10 molar solution would have a pH of -1) |
| What is the definition of a salt? | A salt is any ionic compound whose cation is not H+ and whose anion is not OH-. |
| How do you figure out how many moles of hydrogen ions are in an acid of a certain Molarity for a strong acid? | moles of hydrogen ions = volume in liters X Molarity X # of hydrogen ions that dissociate for that acid (ie - H2SO4 produces twice as many moles of hydrogen ions as HBr) |
| In a titration neutralization reaction, which equations are important to remember? | Since the moles of hydrogen ions will be equal to the moles of hydroxide ions, remember that moles of either is equal to volume X Molarity X # of hydrogen or hydroxide ions liberated. Set them = to each other so that Volume of acid X Molarity of acid X # of hydrogen ions liberated by the acid = Volume of base X Molarity of base X # of hydroxide ions liberated by the base, then solve for the unknown |
| C6H6 in a ring structure is _____. | Benzene |
| What is the molecular formula and shape of benzene? | C6H6, ring |
| In an electrochemical cell, the electrode in the vessel where oxidation occurs is the ______. | anode (remember AN OX and RED CAT) |
| In an electrochemical cell, the electrode in the vessel where reduction occurs is the ______. | cathode (remember AN OX and RED CAT) |
| The electric potential difference for a redox reaction is equal to the ____. | The electric potential difference for a redox reaction is equal to the oxidation potential + the reduction potential (REMEMBER - never multiply the given electric potentials by any coefficients in the chemical equations) |
| A redox reaction will be spontaneous if the electric potential difference for the overall reaction is ___. | greater than zero |