| A | B |
|---|
| This gas is composed of 1 element. Each atom within the molecule makes 1 bond. | Hydrogen, 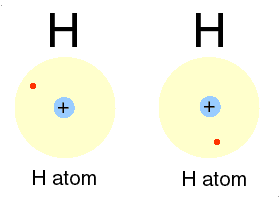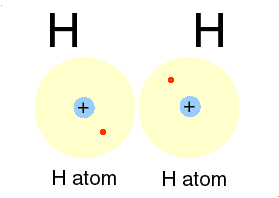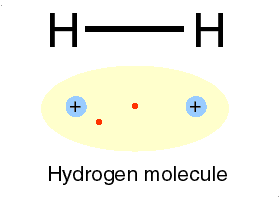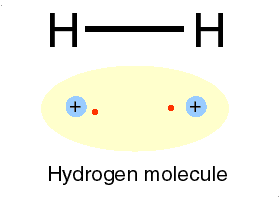 |
| This gas is composed of 1 element. Each atom within the molecule makes 2 bonds. | Oxygen, 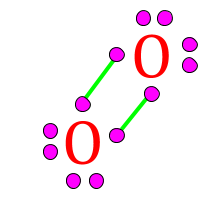 |
| This gas is composed of 1 element. Each atom within the molecule makes 3 bonds. | Nitrogen, 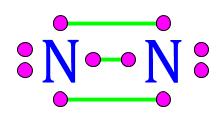 |
| What element makes 4 bonds in this methane molecule? | Carbon, 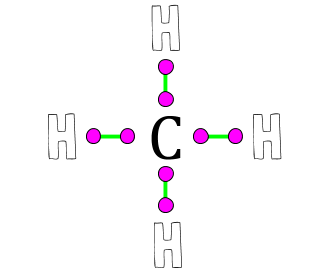 |
| Hydrogen can be found in ____ of the periodic table. | Column 1, 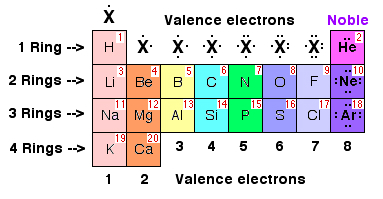 |
| Nitrogen can be found in ____ of the periodic table. | Column 5,  |
| Oxygen can be found in ____ of the periodic table. | Column 6,  |
| Fluorine can be found in ____ of the periodic table. | Column 7,  |
Neon (a noble gas) can be found in ____ of the periodic table.,  | Column 8,  |
| Oxygen is in column 6 of the periodic table, therefore... | ...it makes two bonds., 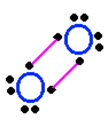 |
| Nitrogen is in column 5 of the periodic table, therefore... | ...it makes three bonds., 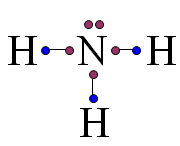 |
| Fluorine is in column 7 of the periodic table, therefore... | ...it makes 1 bond., 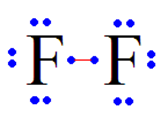 |
| Noble gasses are in column 8 of the periodic table, therefore... | ...they don't make any bonds.,  |
| How many valance electrons does Hydrogen have after it bonds? | It has 2 valence electrons after bonding.,  |
| How many valence electrons do 99% of elements have after they bonds? | They have 8 valence electrons after bonding.,  |
| The _____ Rule reflects observation that bonded atoms tend to have 8 electrons in its outer shell | Octet,  |
| Two exceptions to the Octet Rule are: | Hydrogen and Helium gas; their rings are complete with 2 electrons., 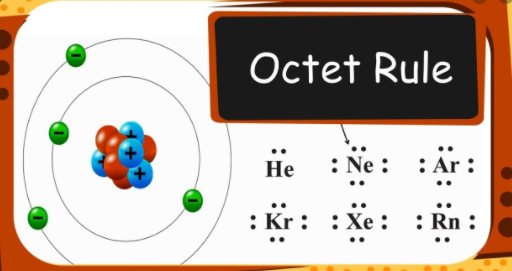 |
| A molecule is... | ...when two or more atoms bond together.,  |
| How many different elements are found in the periodic table? | 90-92 elements are found.,  |
| Which subatomic particle makes elements, elements? | Protons make elements what they are., 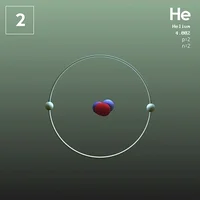 |
Gilbert Lewis discovered the covalent bond and...,  | ... created "Lewis Structures" that show how elements bond., 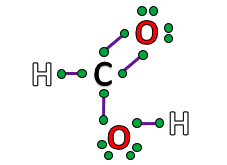 |