| A | B |
|---|
| Combination of features that maximizes electronegativity | a small atomic radius and high ionization energy |
| xxThe Valences electrons have a more negative potential energy, increasing the amount of energy required to move them | xxas the effective nuclear charge is increased with teh same amount of electron shielding |
| why the atomic radius for strontium is greater than the atomic radius for magnesium | strontium has more energy levels and electron shielding than magnesium |
| Comparing the alkali metals to the alkaline earth metals,- | The alkali metals are more electronegative |
| Which trend is represented by graph | graph, 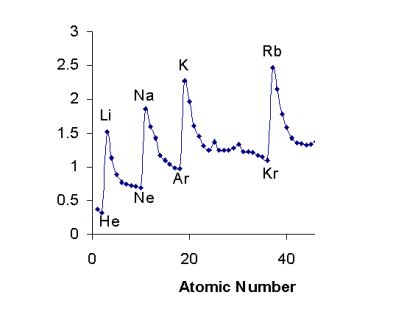 |
| when comparing iodine to bromine, iodine has | more electron shielding |
| An atom with a large atomic radius | has a weak effective nuclear charge and more electron shielding |
| what is true when comparing rubidium to strontium | strontium has a smaller radius and a larger ionization energy |
| Which has greater ionization energy -Sulfur or chlorine? | Chlorine-it has a greater effective nuclear charge to attract valence electrons |
| Which has greatest ionic radius? | S2- |
| Rank elements in order of increasing reactivity from least to most reactive Potassium, gallium, krypton | Kr<Ga<K |
| Which family does Highly electronegativity, highly reactive, and gaseous at room temperature | Halogens |
| All Noble gas except | Low Ionization energies |
So how does a rubber band work? Below (left of the arrow) is how the particles in the rubber band are before stretching. After stretching, the particles align like they are on the right side of the arrow, thus making the rubber band “appear” longer. Based on the picture below, is stretching a physical or chemical change?, 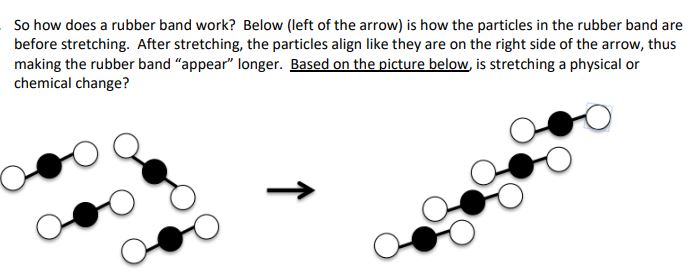 | Physical change, because the molecules changed positions |
Which of the following numbers show where only chemical change occurred? [Note: All circles represent atoms of the same element.], 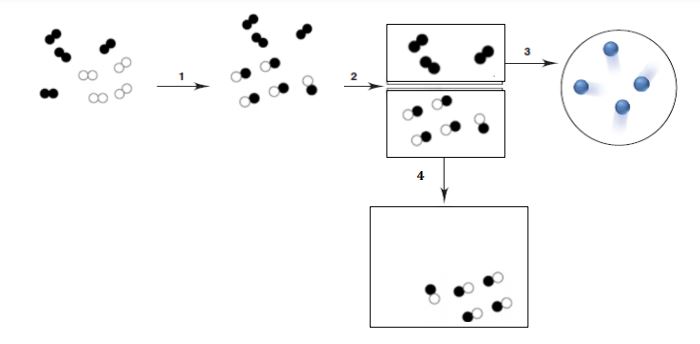 | 3 and 4 |
| Which of the following is an example of a chemical change? | A cold pack is snapped, causing the temperature to suddenly drop. |
| Which has a greater ionization energy, N or P? | Nitrogen has a greater ionization energy than phosphorus. |
| Nitrogen has the same effective nuclear charge as phosphorus (+5). Valence electrons for nitrogen are more shielded than the valence electrons for phosphorus. | Nitrogen has two energy levels (shells) whereas phosphorus has three energy levels (shells). Nitrogen is a smaller atom than phosphorus. |